Session Information
Date: Monday, November 6, 2017
Title: Rheumatoid Arthritis – Clinical Aspects II: Treatment Patterns
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Methotrexate (MTX) remains a cornerstone therapy in the management of rheumatoid arthritis (RA), but patterns of adherence, intolerance, and inadequate response are not well characterized in real-world settings. We compared the characteristics and reasons for MTX discontinuation in patients (pts) with RA who received MTX either as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
Methods: This analysis included pts enrolled in a US RA registry initiating MTX, who were naïve to targeted immune modulators (TIMs; biologics and small molecules). Cox proportional hazards models estimated the hazard ratio (HR) associated with MTX discontinuation and, in a separate analysis, TIM initiation, adjusting for potentially confounding factors. The reasons for MTX continuation were summarized descriptively, conditional on non-missing data.
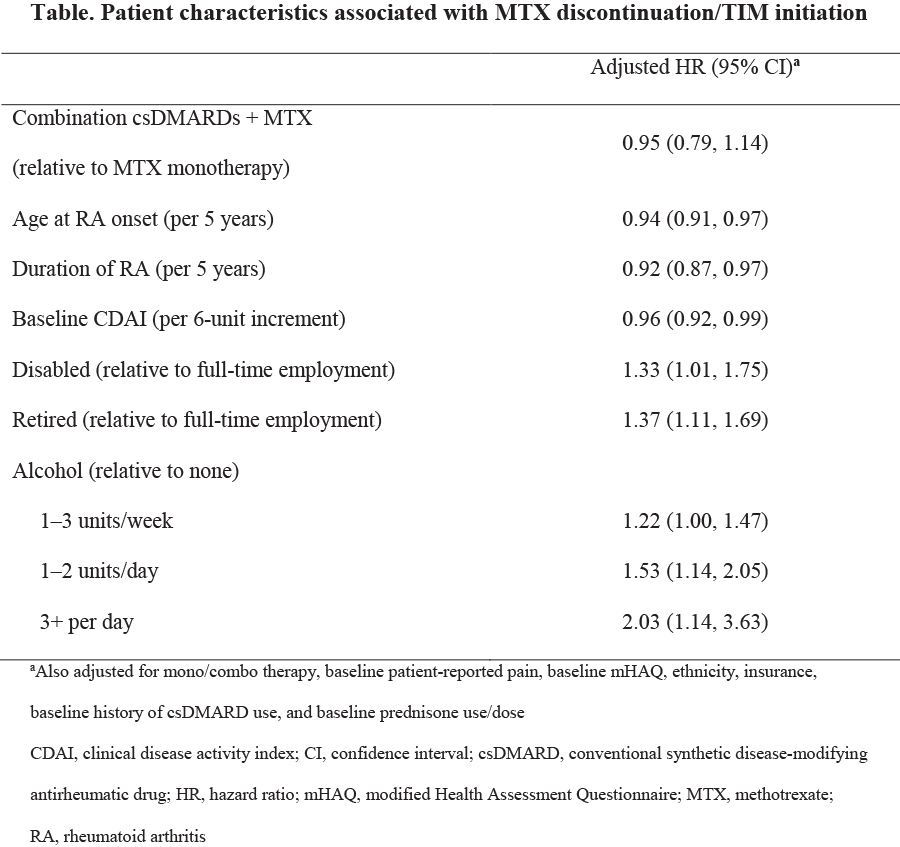
Results: A total of 2144 RA pts initiated MTX (monotherapy: 69%; csDMARD users: 31%). csDMARD users were more likely to be Caucasian, non‑Hispanic, have higher education, be employed full time, and have commercial insurance. Compared with MTX monotherapy, csDMARD users had lower Clinical Disease Activity Index (CDAI) scores (19.6 vs 16.9, p<0.0001), less disability as measured by the modified Health Assessment Questionnaire (mHAQ <0.5; 59% vs 70%, p<0.0001), longer duration of RA (3.8 vs 5.4 years, p<0.0001), and were less likely to be on a higher dose of prednisone (≥10 mg/day; 33% vs 22%, p<0.0001). The proportion of MTX monotherapy pts that discontinued MTX was 24% (1 year), 37% (2 years), and 46% (3 years), and was not significantly different from csDMARD users. The hazard of MTX discontinuation decreased as age at RA onset, duration of disease, and baseline CDAI increased (Table). After adjusting for potential confounding variables, an increased hazard of MTX discontinuation was associated with being disabled, retired, or regular alcohol use. MTX discontinuation was less likely in pts with older age, longer duration of RA, or higher baseline CDAI (Table). At 12 months, 27% of MTX monotherapy and 18% of csDMARD users had initiated a TIM. MTX monotherapy users were 39% more likely to have initiated a TIM by Month 12 compared with csDMARD users (HR 1.39; 95% CI 1.19, 1.59). However, after multivariable adjustment, the association between MTX monotherapy and TIM initiation was attenuated (HR 1.15; 95% CI 0.94, 1.41). The most common reasons reported for discontinuing MTX were safety/tolerability (48%) and other reasons (29%), rather than lack of efficacy (15%).
Conclusion: Despite the well-recognized role of MTX in RA management, approximately 30% of pts with RA discontinue MTX within 1–2 years after initiation. Strategies are required to better identify pts with suboptimal adherence to MTX and predict those most likely to not tolerate MTX, in order to optimize overall RA treatment.
To cite this abstract in AMA style:
Curtis JR, Wallenstein G, Takiya L, Gruben D, Chen C, Shan Y, Blachley T, Dandreo KJ, Kremer J. Patterns of Methotrexate Use and Discontinuation in a U.S. Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/patterns-of-methotrexate-use-and-discontinuation-in-a-u-s-rheumatoid-arthritis-registry/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-methotrexate-use-and-discontinuation-in-a-u-s-rheumatoid-arthritis-registry/