Session Information
Date: Monday, November 6, 2017
Title: Plenary Session II
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Use of biologic therapies for rheumatoid arthritis (RA) in pregnancy is common. There is theoretical concern that these medications could interfere with postnatal immune function in the infant.
Methods: The Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project enrolled pregnant women from U.S. or Canada with or without RA in a prospective observational cohort study. Women were followed with 3-4 telephone interviews; medical records were abstracted from the delivery hospital, obstetric and specialty providers; follow-up data were obtained from the pediatrician through 1 year of age. Maternal use of biologic medications including start/stop dates was captured, and questions were asked about serious/opportunistic infections in the infant. These were defined as an infection requiring hospitalization or any of the following: neonatal sepsis, invasive fungal infection, x-ray proven pneumonia, meningitis, bacteremia, pneumocystis, septic arthritis, osteomyelitis, tuberculosis, herpes, listeria, legionella, mycobacteria, systemic cytomegalovirus, or abscess. We estimated relative risks (RR) and 95% confidence intervals (CI) using generalized estimating equations. We further stratified comparisons by gestational timing of last dose of the biologic after 24 and 32 weeks gestation, respectively.
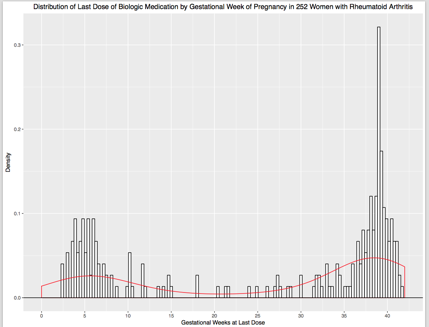
Results: Between 2004-2016, 1,184 pregnancies ending in live born infants, including 73 infants of twin pregnancies, were followed to 1 year postpartum (252 with RA treated with a biologic in pregnancy, 463 with RA but no treatment with a biologic in pregnancy, and 469 with no chronic diseases). Of those in the RA biologic group, 97 (38.4%) took their last dose in the 1st or 2nd trimester and 155 (61.5%) received their last dose in the 3rd trimester (Figure). Serious or opportunistic infections were reported in 7/252 (2.8%) of infants born to women with RA treated with a biologic, 18/463 (3.9%) of infants born to women with RA not treated with a biologic (RR 0.71, 95% CI 0.30, 1.71), and 12/469 (2.6%) of those infants whose mothers had no chronic diseases (RR 1.09, 95% CI 0.43, 2.72). Restricting the group treated with a biologic to women whose last dose was sometime after 24 weeks gestation, 6/155 (3.9%) reported infant infections in the biologics group compared to 18/463 (3.9%) in the RA comparison group (RR 1.00, 95% CI 0.40, 2.48). Further stratification at 32 weeks gestation resulted in infections reported in 5/143 (3.5%) in the RA biologics group compared to the 3.9% in the RA untreated group (RR 0.90, 95% CI 0.34, 2.39).
Conclusion: We found no evidence of increased risk of serious/opportunistic infections in infants in this sample. These data are reassuring for pregnant women who require treatment with a biologic medication. However, it is possible that less serious infections occur more frequently, and further research is needed.
To cite this abstract in AMA style:
Chambers CD, Johnson DL, Luo Y, Xu R, Jones KL. Serious or Opportunistic Infections in Infants Born to Pregnant Women with Rheumatoid Arthritis and Treated with a Biologic Medication [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/serious-or-opportunistic-infections-in-infants-born-to-pregnant-women-with-rheumatoid-arthritis-and-treated-with-a-biologic-medication/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serious-or-opportunistic-infections-in-infants-born-to-pregnant-women-with-rheumatoid-arthritis-and-treated-with-a-biologic-medication/