Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To better understand the real-world efficacy of biologics for the treatment of psoriatic arthritis (PsA), there is a need to evaluate the persistence and adherence with biologics among patients with PsA seen in routine clinical practice. This study described the patient demographics, disease characteristics and treatment patterns of patients with PsA who initiated a subcutaneously administered (SC) biologic in a US claims database.
Methods: Patients with ≥ 1 pharmacy claim for an FDA-approved SC biologic between 01/15/2016 and 01/31/2017 were identified from the Truven Health Analytics MarketScan Commercial and Medicare Supplemental Databases. Eligible patients were aged ≥ 18 years at the time of biologic initiation (index date) and were continuously enrolled with medical and pharmacy claims ≥ 12 months prior to (baseline period) and ≥ 6 months after the index date. Patients had ≥ 1 PsA diagnosis (ICD-9-CM 696.0 or ICD-10-CM L40.5x) and no pharmacy claims for the index biologic during the baseline period. Patient demographics were assessed at the index date; clinical characteristics and treatment history were examined during the baseline period. Biologic discontinuation, number of days persistent and adherence (proportion of days covered) with the index biologic were evaluated over the 6-month follow-up period.
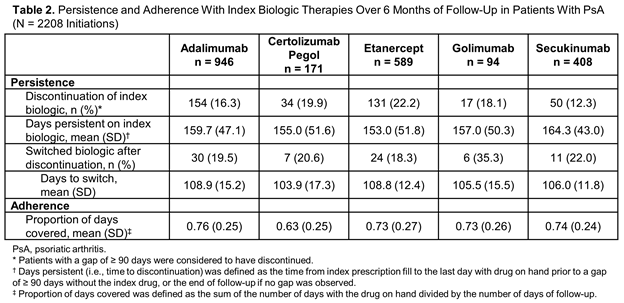
Results: A total of 2208 eligible SC biologic initiations were identified in patients with PsA, including adalimumab (n = 946), certolizumab pegol (n = 171), etanercept (n = 589), golimumab (n = 94) and secukinumab (n = 408), with mean follow-up of 253 to 275 days. Demographics and clinical characteristics were similar across biologic groups (Table 1). A lower proportion of patients initiating adalimumab or etanercept had prior biologic use (28% and 37%, respectively) compared with certolizumab pegol (72%), golimumab (68%) and secukinumab (76%). The 6-month discontinuation rate was lowest with secukinumab (12%), followed by adalimumab (16%), golimumab (18%), certolizumab pegol (20%) and etanercept (22%). Mean days persistent on the index biologic ranged from 153 days (etanercept) to 164 days (secukinumab). The mean proportion of days covered was similar among secukinumab, adalimumab, etanercept and golimumab (0.73-0.76) and lowest for certolizumab pegol (0.63) (Table 2).
Conclusion: Patients who initiated secukinumab had the lowest discontinuation rate and highest number of days persistent over 6 months compared with the other SC biologics assessed.
To cite this abstract in AMA style:
Oelke KR, Garg R, Hur P, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Palmer JB. Characteristics and Treatment Patterns Among Patients with Psoriatic Arthritis Initiating Subcutaneously Administered Biologics: Descriptive Analyses from a US Claims Database [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-among-patients-with-psoriatic-arthritis-initiating-subcutaneously-administered-biologics-descriptive-analyses-from-a-us-claims-database/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-among-patients-with-psoriatic-arthritis-initiating-subcutaneously-administered-biologics-descriptive-analyses-from-a-us-claims-database/