Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the effectiveness and safety of Certolizumab Pegol (CZP) in a real word setting in Psoriatic Arthritis (PsA) patients.
Methods: Multicentric cohort of PsA patients treated with CZP according to routine clinical practice. Study approved by local Ethics Committee. Maximum time of observation is 12 months. Effectiveness variables: SJC, TJC, PtGA, PhGA and DAS28-CRP. Safety variables: discontinuation rate.
Results: 262 patients with PsA were included: 43.5% male, mean (SD) age 49.9 (±11.9) years, median disease duration 5.0 (0, 40.2) years, 14.9% of patients were HLAB27 positive, BMI (kg/m2) 26.9 (±4.7) and never smokers 70.3%. Regarding extra-articular manifestations ever: Psoriasis (90%; PASI≥10 4.9%), enthesitis (44.4%), dactilytis (41.9%), nail disease (32%), inflammatory bowel diseases (4.9%). 37.3% of the PsA patients had erosions and a 3% arthritis mutillans (N=8). 48.9% patients received 1 prior csDMARDs and 52.1% at least 2 csDMARDs. Prior bDMARD received (28.4% none; 38.1% 1, 33.5% ≥2). 29.6% of PsA patients received CZP in monotherapy. Mean duration on treatment with CZP 10 m (78.2%).
Statistically significant differences in SJC, TJC and DAS28[CRP] were observed at the last visit comparing with baseline (Table 1). In the last observation, 47.1% of the patients had a DAS28 response (reduction of ≥1.2 from baseline). The percentage of patients with enthesitis at baseline (25.4%) was reduced to 9.5% at the end of the observation; 73.2% of the patients had a total resolution of the enthesitis (MASES=0). The percentage of patients with dactilytis at baseline (29.1%) decreased to 8.6% in the last observation; 82.5% of these patients had a total resolution of the dactilytis.
Table 1. Evolution of clinical variables of activity
|
|
Baseline |
Last observation |
|
DAS28-crp; mean±SD |
4.6±0.9 (N=210) |
3.8±1.0 (N=132)* |
|
TJC evolution; mean±SD |
7.2±5.1 (N=228) |
4.0±4.0 (N=188)* |
|
SJC evolution; mean±SD |
5.0±3.7 (N=212) |
2.8±2.8 (N=137)* |
*p<0,001, Wilcoxon’s test
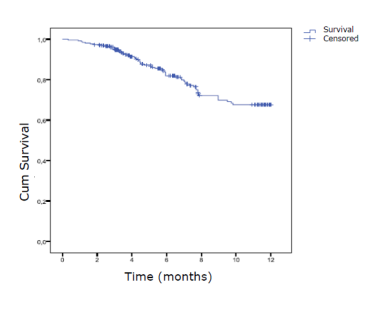
The drug survival of CZP is 78.2% (Figure1), and similar retention rates were observed independently if CZP were used as monotherapy (79.2%) or in combination with DMARDs (78.9%).
Figure 1.
262 patients were included in the safety analysis, 21.8% withdrawn treatment: 12.6% due to lack of efficacy, 5.3% due to intolerance and 3.8% other reasons.
Conclusion: Real life experience from this nationwide study, demonstrated the effectiveness and safety of CZP in PsA patients (bionaïve only: 28.4%), with a significant reduction of DAS28, enthesitis and dactilytis. CZP survival seems to be similar regardless of csDMARDs concomitant use.
This publication has been possible thanks to a grant of UCB Pharma to the technical companies which have been managing the data collection and the statistical analyses. The results are independent of UCB Pharma
To cite this abstract in AMA style:
Conesa A, Fernández M, Expósito R, Campos J, Lamua JR, Navarro MDP, Rubio-Muñoz P, Ahijado-Guzman P, Gonzalez CM. Certolizumab Pegol Effectiveness and Retention Rate in Psoriatic Arthritis. Real Life Data [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/certolizumab-pegol-effectiveness-and-retention-rate-in-psoriatic-arthritis-real-life-data/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/certolizumab-pegol-effectiveness-and-retention-rate-in-psoriatic-arthritis-real-life-data/