Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
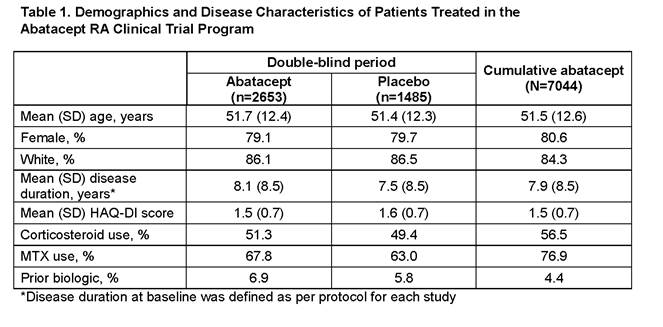
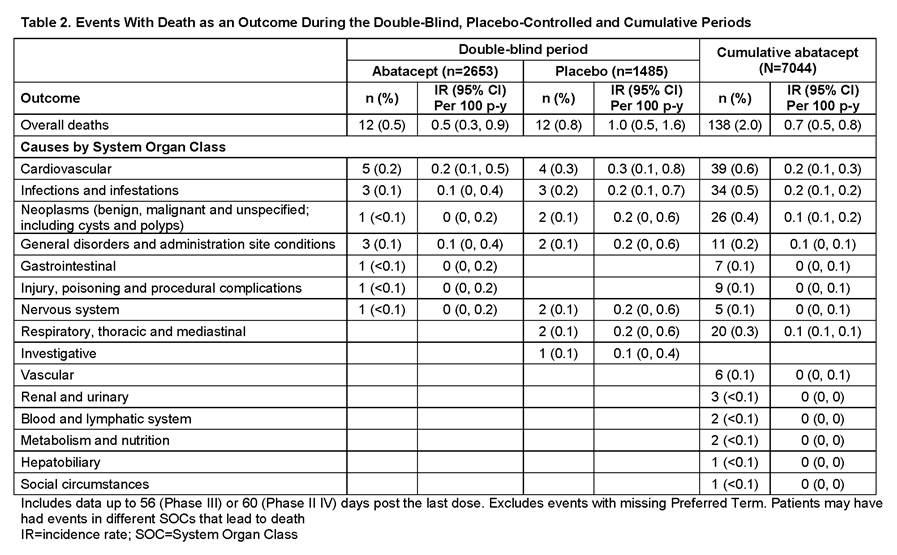
Conclusion: The data from this ongoing assessment of abatacept safety are consistent with the current known benefit–risk profile of abatacept. In the DB phases, the overall IRs of death (95% CI) were 0.5 (0.3, 0.9) on abatacept and 1.0 (0.5, 1.6) on plabebo. In the cumulative abatacept population, IR was 0.7 (0.5, 0.8), similar to both the DB population and published literature.
1. Sihvonen S, et al. J Rheumatol 2006;33:1740–6.
2. Choi HK, et al. Lancet 2002;359:1173–7.
3. Jacobsson LT, et al. Ann Rheum Dis 2007;66:670–5.
To cite this abstract in AMA style:
Fleming D, Simon T, Torbeyns A, Meier-Kriesche U, Johnsen A. Incidence Rates of Adverse Events with Death As an Outcome during Abatacept Treatment in RA: Results from an Integrated Data Analysis from 16 Clinical Trials [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/incidence-rates-of-adverse-events-with-death-as-an-outcome-during-abatacept-treatment-in-ra-results-from-an-integrated-data-analysis-from-16-clinical-trials/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-rates-of-adverse-events-with-death-as-an-outcome-during-abatacept-treatment-in-ra-results-from-an-integrated-data-analysis-from-16-clinical-trials/