Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: There are limited data regarding the impact of tocilizumab (TCZ) treatment on prednisone use in patients with rheumatoid arthritis (RA). The objective of this study was to examine prednisone use among US patients with RA who initiated TCZ in routine clinical practice, overall and stratified by initiation of TCZ as monotherapy (TCZ mono) or in combination with a conventional synthetic DMARD (csDMARD; TCZ combo).
Methods: TCZ-naïve patients enrolled in the Corrona RA registry (NCT01402661) who initiated TCZ on or after January 1, 2010; had a 12-month follow-up visit without discontinuation of TCZ and had prednisone dose information available at the time of TCZ initiation (baseline) and follow-up were included. Outcomes included the proportion of patients with changes in prednisone use (initiation, discontinuation, dose escalation or reduction ≥ 5 mg) and change in Clinical Disease Activity Index (CDAI) score at 12 months. Outcomes were assessed in the overall population and stratified by initiation of TCZ mono or TCZ combo. Baseline patient characteristics were compared between the TCZ mono and TCZ combo groups using 2-sample t tests or Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher exact tests for categorical variables.
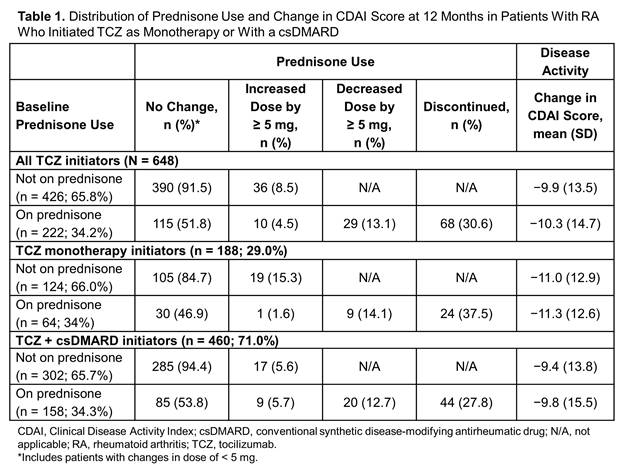
Results: Of the 648 eligible patients, 29% initiated TCZ mono and 71% initiated TCZ combo. Patients who initiated TCZ mono were older (mean age, 60.5 vs 57.9 years; P = 0.01), weighed less (mean weight, 174.0 vs 184.4 pounds; P = 0.01), were less likely to have private insurance (71.8% vs 79.3%; P = 0.04) and had used fewer csDMARDs (P < 0.001) and tumor necrosis factor inhibitors (P = 0.03) compared with TCZ combo. At baseline, 222 patients (34.3%) were receiving prednisone and 426 (65.7%) were not. Changes in prednisone use, including discontinuation and dose reduction ≥ 5 mg, were observed within 6 months after TCZ initiation. Of the patients on prednisone at baseline, 30.6% had discontinued prednisone and 13.1% had decreased the dose after 12 months of TCZ therapy (Table 1). Of those not on prednisone at baseline, only 8.5% initiated prednisone over 12 months of TCZ therapy (Table 1). Changes in prednisone use were not different between TCZ mono and TCZ combo, and there were no differences in CDAI improvement between patients using prednisone vs not using prednisone at baseline, overall or stratified by TCZ mono vs TCZ combo (Table 1).
Conclusion: A considerable proportion of patients initiating TCZ were able to discontinue or lower the dose of prednisone over 12 months. Changes in prednisone use were observed within 6 months after TCZ initiation, were similar regardless of whether TCZ was administered as monotherapy or in combination with csDMARDs and did not result in differences in the observed decrease in disease activity.
To cite this abstract in AMA style:
Pappas DA, Etzel CJ, Best J, Zlotnick S, Blachley T, Persuitte G, Kremer J. Patterns of Prednisone Use in US Patients with Rheumatoid Arthritis Initiating Treatment with Tocilizumab in Routine Clinical Practice [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/patterns-of-prednisone-use-in-us-patients-with-rheumatoid-arthritis-initiating-treatment-with-tocilizumab-in-routine-clinical-practice/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-prednisone-use-in-us-patients-with-rheumatoid-arthritis-initiating-treatment-with-tocilizumab-in-routine-clinical-practice/