Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Steroids are used to reduce inflammation and pain among rheumatoid arthritis (RA) patients, but they can cause harmful side effects, and in some patients, difficulty to discontinue/taper the dose. Adalimumab (ADA) has been shown to be effective in treating RA patients with a well-established safety profile. The objective of this study was to determine if patients on ADA treatment were able to discontinue or lower the dose of steroids post-ADA initiation.
Methods: Biologic naïve RA patients who initiated ADA after 2008, and with at least 6 months of continuous therapy were included in the study. The difference in mean steroid dose from ADA initiation (baseline) to 6 months was evaluated for all ADA initiators and by type of therapy (monotherapy/combination therapy with methotrexate). Paired t-tests were used to compare changes. The distribution of steroid dose categories (0 mg, >0 to 5 mg, >5 to 10 mg, >10 to 15 mg and >15 mg) at baseline and 6 months was also described and stratified by ADA monotherapy and combination therapy. Descriptive characteristics at baseline were examined; appropriate tests of comparisons (1-way ANOVA/chi-square/Kruskal-Wallis) were used to compare across type of therapy.
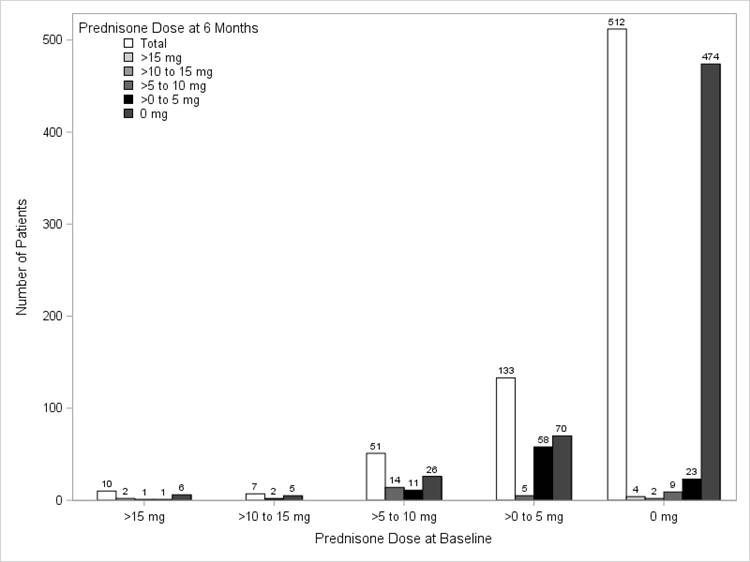
Results: Among 713 biologic naïve ADA initiators who were eligible to participate in the study, 239 (34%) used steroids during the 6 month follow up period: 109 at initiation only, 94 at initiation and follow-up, and 38 at follow-up only. The mean (SD) age of the study population was 55.4 (12.3) years, 73% were female, and the mean (SD) disease duration was 5.1 (7.4) years. Mean (SD) clinical disease activity index at baseline was 20.1 (14.9), with ~70% in moderate/severe disease activity (CDAI>10) and a mean (SD) functional disability index score of 0.5 (0.5) as measured by the modified health assessment questionnaire. Mean steroid dose at baseline was 6.1 mg (6.1) and over the 6 months of therapy, the steroid dose reduced to a mean (SD) dose of 3.6 mg (5.0), with a mean (SD) change of 2.5 mg (8.0) (p<0.0001). Similar dose reductions were seen in ADA monotherapy and combination therapy groups (data not shown). As seen in the table below, approximately 53% of the 201 patients who were on steroids at ADA initiation were able to discontinue the steroid by 6 months.
Conclusion: In this real world study of ADA initiators, approximately half of the patients who received concomitant steroids at initiation were able to discontinue the steroid by 6 months. For those who continued steroids at 6 months, patients were on average able to significantly reduce their dose.
Figure. Distribution of steroid dose at ADA initiation vs steroid dose at the 6-month follow-up visit for all initiators (N=239)
To cite this abstract in AMA style:
Pappas DA, Karki C, Litman HJ, Blachley T, Suboticki JL, Griffith J, Kremer J. Impact of Adalimumab on Prednisone Use in Patients with Rheumatoid Arthritis in a Real World Setting – Results from the Corrona Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/impact-of-adalimumab-on-prednisone-use-in-patients-with-rheumatoid-arthritis-in-a-real-world-setting-results-from-the-corrona-registry/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-adalimumab-on-prednisone-use-in-patients-with-rheumatoid-arthritis-in-a-real-world-setting-results-from-the-corrona-registry/