Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Low dose glucocorticoids (GCs) are recommended in combination with ≥1 synthetic DMARDs as part of the initial treatment strategy in rheumatoid arthritis (RA), with taper recommended as quickly as is clinically feasible1. The purpose of this analysis was to evaluate the ability of patients (pts) with early RA receiving MTX monotherapy or adalimumab (ADA)+MTX to taper and discontinue low-dose GCs and associated impact on outcomes.
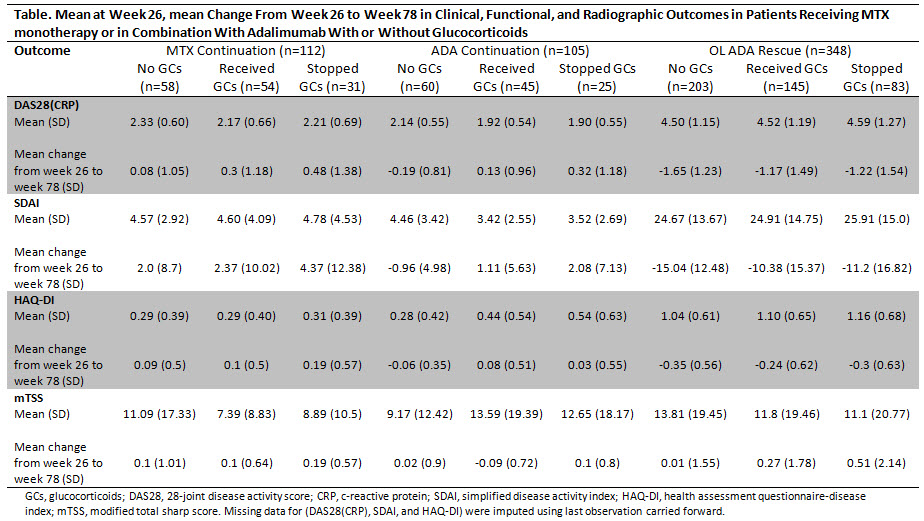
Methods: This post hoc analysis included MTX naïve pts receiving PBO+MTX or ADA+MTX in the double-period OPTIMA trial. Pts achieving stable low disease activity (sLDA; DAS28 <3.2 at wks 22 and 26) during the first 26 wks (P1) continued blinded therapy (MTX, ADA continuation) for an additional 52 wks (P2). PBO+MTX pts not achieving sLDA were offered open-label (OL) ADA+MTX in P2 (OL ADA rescue). Pts receiving GCs (≤10 mg/d) at baseline (BL) continued a stable dose through P1; tapering ≤1mg/EOW was permitted in P2. Pts were categorized by GC use in each treatment group. The proportions of pts discontinuing GC were summarized. Outcomes were assessed as change from wk 26 to wk 78. Adverse events (AEs) were summarized.
Results: Of the 926 pts who entered P2 (RA disease duration ~4 months), 207/460 (45%) and 188/466 (40%) in the initial PBO+MTX and ADA+MTX groups, respectively, had GC treatment at BL. BL characteristics appeared similar between pts receiving and not receiving GCs. In P1, the proportions of pts achieving sLDA between those receiving and not receiving GCs in the PBO+MTX group were similar (51% without and 49% with GCs), while in the ADA+MTX group a higher proportion of pts achieved sLDA without GCs (58% without vs 42% with GCs). At the beginning of P2, 54/112 (48%) and 45/105 (43%) pts in the MTX and ADA continuation groups, respectively, were on GCs; 145/348 (42%) pts in the OL ADA rescue group received GCs. Of these pts, equal proportions [57% (n=31), 56% (n=25), and 57% (n=83) from the MTX continuation, ADA continuation, and OL ADA rescue groups, respectively] discontinued GCs during P2. Although slight increases in disease activity were observed, GC discontinuation across the different groups did not result in clinically meaningful changes through wk 78 (Table). Within the 3 P2 treatment groups, the AE profile appeared similar between those receiving and not receiving GCs.
Conclusion: Approximately 60% of early RA pts receiving PBO+MTX or ADA+MTX along with GCs whose treatment was modified based on attainment of sLDA were able to discontinue their GC use without clinically meaningful worsening in disease activity, structural damage or decreases in function. As the current analysis is limited by its retrospective, uncontrolled nature, further studies are needed to prospectively evaluate the ability of pts with early RA to taper or discontinue GCs.
Reference:
1. Smolen JS, et al. Ann Rheum Dis 2017;76(6):960-977.
To cite this abstract in AMA style:
Smolen JS, Sunkureddi P, Anderson JK, Griffith J, Jiang D, Chen K, Suboticki JL, Kavanaugh A. The Ability of Patients with Early Rheumatoid Arthritis to Taper Low-Dose Glucocorticoids on Methotrexate Monotherapy and in Combination with Adalimumab [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-ability-of-patients-with-early-rheumatoid-arthritis-to-taper-low-dose-glucocorticoids-on-methotrexate-monotherapy-and-in-combination-with-adalimumab/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-ability-of-patients-with-early-rheumatoid-arthritis-to-taper-low-dose-glucocorticoids-on-methotrexate-monotherapy-and-in-combination-with-adalimumab/