Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients (pts) with rheumatoid arthritis (RA), treat-to-target recommendations call for adjustment of treatment if a target is not met within 3-6 months (mths) of initiation. While some pts continue therapy beyond 3-6 mths despite not achieving the target, it’s unclear if they still can achieve the target, and how the timing of target attainment impacts long-term outcomes.
Objective: To evaluate clinical, functional, and radiographic outcomes based on initial time to low disease activity (LDA) attainment among early RA pts who are naïve to MTX, or are MTX-insufficient responders (MTX-IR).
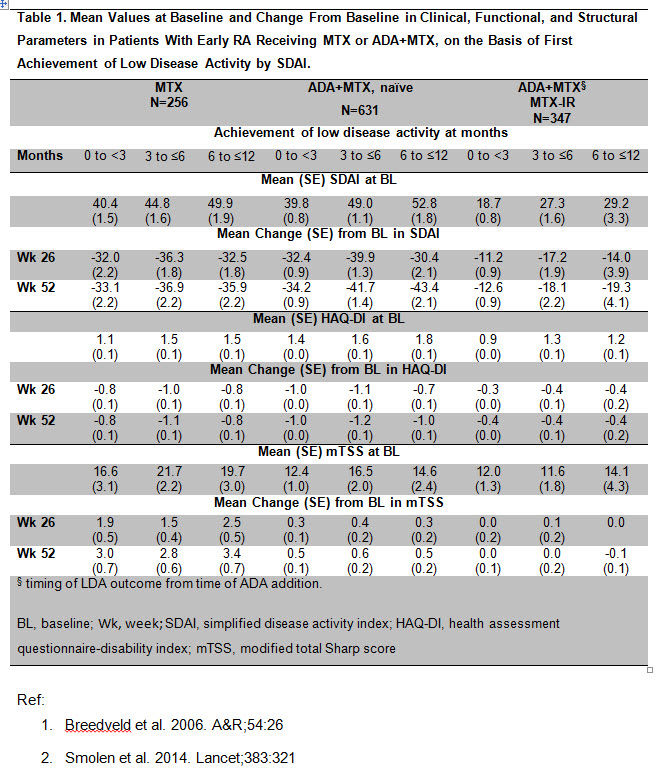
Methods: This post hoc analysis included pts receiving MTX in monotherapy or in combination with adalimumab (ADA) in 2 randomized, controlled trials (RCTs) of MTX-naïve pts with early RA: PREMIER included a 104-week (wk) RCT1; OPTIMA included a 26 wk RCT followed by treatment adjustments based on a target of LDA at wks 22 and 262. Pts not achieving stable LDA received open-label (OL) ADA+MTX for an additional 52 wks (MTX- IR). Pts were subgrouped by treatment and time to first LDA event [SDAI ≤11: 0-<3 mths, 3-≤6 mths, >6-≤12 mths]. The following were summarized for each subgroup: mean values and change from baseline (Δ) in SDAI, HAQ-DI and modified total Sharp score (mTSS). The proportions of pts achieving SDAI remission (REM) at 1 year (yr) were assessed.
Results: Roughly equal proportions of pts on MTX alone experienced their first LDA response between 0-<3 mths (21%), 3-≤6 mths (21%), and >6-≤12 mths (17%). More pts on ADA+MTX experienced LDA within 3 mths (0-<3: 45% and 56% for MTX-naïve and MTX-IR backgrounds, respectively), with smaller proportions in the 3-≤6 mths (19% for both MTX-naïve and –IR backgrounds), and >6-≤12 mths groups (14% and 4% for MTX-naïve and MTX-IR backgrounds). Approximately 50% of the 0–<3 mth group across treatments achieved SDAI REM at 1 yr. Interestingly, 10%, 14%, and 8% of the MTX, ADA+MTX (MTX-naïve), and ADA+MTX (MTX-IR) pts who first experienced LDA after 6 mths were in SDAI REM at 1yr.
Among MTX-naïve pts, pts on ADA+MTX had greater ΔHAQ and smaller ΔmTSS than pts on MTX alone at Wks 26 and 52 (Table 1). Regardless of their time to first SDAI LDA response, pts on MTX monotherapy or ADA+MTX experienced comparable improvements in SDAI, HAQ-DI and comparable ΔmTSS at Wks 26 and 52.
Conclusion: Pts on ADA+MTX achieved a first SDAI LDA response earlier than pts on MTX monotherapy, regardless of whether they were MTX-naïve or MTX-IR. More pts with a very early response went on to achieve SDAI REM at 1 yr. However, pts with a longer time (>6 mths) to their first SDAI LDA response had comparable clinical, functional and radiographic outcomes compared to pts who responded earlier (within 3 or 6 mths). Therefore, achieving a clinical response in the direction of the treatment target, even if not yet achieving it, may be sufficient to continue therapy in appropriate pts.
To cite this abstract in AMA style:
Smolen JS, Bu X, Wang X, Suboticki JL, Kavanaugh A. Characteristics of Patients with Early Rheumatoid Arthritis Who Have a Delayed Response to Treatment with Methotrexate in Monotherapy or in Combination with Adalimumab [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/characteristics-of-patients-with-early-rheumatoid-arthritis-who-have-a-delayed-response-to-treatment-with-methotrexate-in-monotherapy-or-in-combination-with-adalimumab/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-of-patients-with-early-rheumatoid-arthritis-who-have-a-delayed-response-to-treatment-with-methotrexate-in-monotherapy-or-in-combination-with-adalimumab/