Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-tumor necrosis factor medications (anti-TNFs) are effective in controlling chronic inflammatory diseases, but information about their use and safety in pregnancy is limited. Consequently, anti-TNFs are often discontinued early in gestation. Certolizumab pegol (CZP), a PEGylated, Fc-free anti-TNF approved for treatment of rheumatic diseases and/or Crohn’s disease, has no active placental transfer. This project provides information on pregnancy outcomes in women receiving CZP, especially those with early pregnancy exposure.
Methods: Prospective and retrospective data on maternal CZP exposure, including timing and duration, outcomes, comorbidities, and major malformations were extracted from the UCB Pharma safety database through March 6, 2017. This analysis was limited to prospective reports to avoid bias associated with retrospective submissions. Numbers of live births, miscarriages, elective abortions, stillbirths, and major congenital malformations were ascertained.
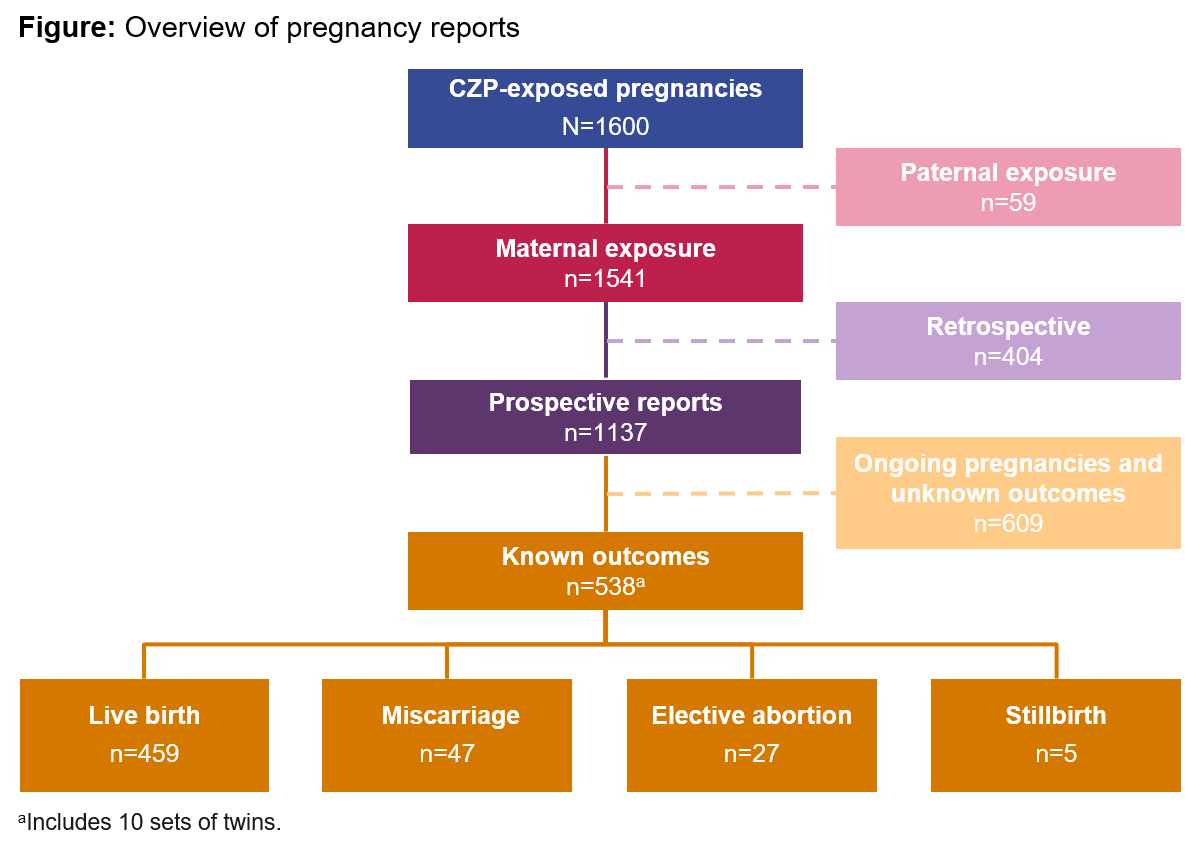
Results: From a total of 1541 maternal CZP-exposed pregnancies, 1137 were reported prospectively, of which 528 pregnancies (including 10 twins) had 538 known outcomes: 459 live births (85%), 47 miscarriages (9%), 27 elective abortions (5%), and 5 stillbirths (1%) (Figure). Of the 459 live births, 8 (1.7%) cases of major congenital malformations were reported (vesicoureteral reflux, club foot, congenital heart disease, cerebral ventricle dilatation, polydactyly, anal fistula, accessory auricle, and hydronephrosis). Of the 528 prospective pregnancies with known outcomes, 436 (83%) were exposed during the 1st trimester, when most organogenesis occurs; 202 out of the 436 were exposed during the entire pregnancy.
Conclusion: This analysis represents the largest cohort of pregnant women exposed to an anti-TNF for management of chronic inflammatory diseases. Although these data should be interpreted with caution, there is no observed increased risk of major malformations or other adverse pregnancy outcomes with CZP use compared to baseline, as reported for the US general population by the Centers for Disease Control and Prevention (3%). The data are reassuring for women of childbearing age considering treatment with CZP.
To cite this abstract in AMA style:
Clowse MEB, Scheuerle AE, Chambers CD, Afzali A, Kimball A, Cush JJ, Cooney M, Shaughnessy L, Vanderkelen M, Förger F. Characteristics and Outcomes of Prospectively Reported Pregnancies Exposed to Certolizumab Pegol from a Safety Database [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/characteristics-and-outcomes-of-prospectively-reported-pregnancies-exposed-to-certolizumab-pegol-from-a-safety-database-2/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-outcomes-of-prospectively-reported-pregnancies-exposed-to-certolizumab-pegol-from-a-safety-database-2/