Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Checkpoint inhibitor immunotherapy has fundamentally changed the treatment for an actively expanding list of cancers, however its use is associated with immune related adverse events (irAEs). Rheumatologic manifestations of irAEs remain incompletely characterized and poorly understood. A recently published case series suggested that immunotherapy-induced arthritis is an aggressive process requiring high dose corticosteroids.
Methods: This was a retrospective chart review of all patients with musculoskeletal irAEs first seen by one of the authors prior to October 2016. All patients had been treated for a malignancy with immune checkpoint inhibitors targeting PD-1 (nivolumab, pembrolizumab), PD-L1 (durvalumab) and/or CTLA-4 (ipilimumab, tremelimumab) at Memorial Sloan Kettering Cancer Center between 2013 and 2016.
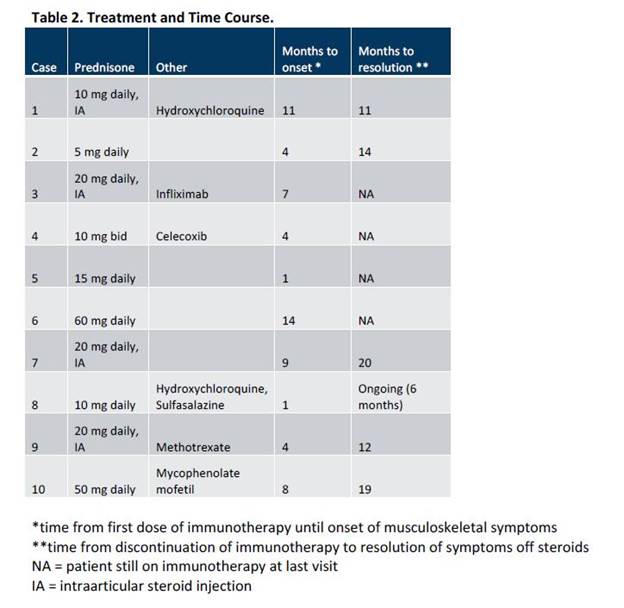
Results: We identified 10 patients with a mean (± standard deviation) age 63.2 (± 9.7) years (Table 1). Seven patients were treated with a combination of checkpoint inhibitors and three with nivolumab monotherapy. Four patients developed inflammatory polyarthritis, four oligoarthritis and two tenosynovitis. Six were ANA positive and two had anti-CCP antibodies. Mean time from the first dose of immunotherapy until joint involvement was 6.3 (± 4.3) months (Table 2). Of the two patients with the shortest interval between immunotherapy and the onset of arthritis, one had had mild polymyalgia rheumatic (PMR)-like symptoms prior to the initiation of immunotherapy, and the other had a sister with rheumatoid arthritis (RA). All 10 patients were treated with systemic corticosteroids, and eight required 20 mg or less per day of prednisone. Five patients received steroid-sparing agents. The mean time until resolution of joint symptoms after the last dose of immunotherapy was 15.2 (± 4.1) months.
Conclusion: Patients experiencing musculoskeletal irAEs can have a wide variety of manifestations ranging from oligoarthritis and tenosynovitis, to PMR, to a polyarthritis consistent with RA. The onset of arthritis appears to be relatively late compared to other irAEs. Musculoskeletal symptoms can last more than a year after the last dose of immunotherapy, however they can generally be managed with low doses of corticosteroids.
To cite this abstract in AMA style:
Smith MH, Bass AR. Arthritis after Cancer Immunotherapy: Symptom Duration and Treatment Response [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/arthritis-after-cancer-immunotherapy-symptom-duration-and-treatment-response/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/arthritis-after-cancer-immunotherapy-symptom-duration-and-treatment-response/