Session Information
Date: Monday, November 6, 2017
Title: Health Services Research Poster II: Osteoarthritis and Rheumatoid Arthritis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Treatment with biological DMARDs (bDMARD) and targeted synthetic DMARDs (tsDMARD) has led to improved outcomes for chronic rheumatic diseases. Current treat-to-target (T2T) strategy relies on quick escalation in therapy to achieve disease control. A delay in escalation of therapy may impact clinical outcomes adversely. Insurance prior authorization, required for specialty DMARD approval, represents a significant bottleneck to care. Clinical pharmacists embedded in a collaborative clinical team could optimize rheumatologic care by shortening time to specialty medication authorization. Our aim was to assess the impact of pharmacy support on specialty DMARD approval in an academic rheumatology clinic.
Methods: We performed a retrospective review of patient records followed in the Rheumatology Clinics at the University of Kentucky who were prescribed specialty medications for RA, PsA and AS. All patients were diagnosed by clinical rheumatologists and met ACR, CASPAR or ASAS criteria respectively. We included 8 bDMARDs and 2 tsDMARDs currently prescribed. We collected data during two 6 month periods from prescriptions written pre-pharmacy (1/2014-6/2014) and post-pharmacy establishment (1/2016-6/2016). We excluded the transition to pharmacist period of 7/2014-12/2015. Our primary outcome was the time to authorization defined as time (in days) between rheumatologist’s prescribing and pharmacist’s completion of authorization for a given specialty DMARD. We compared the mean time to authorization pre- and post-pharmacist establishment in the rheumatology clinic. Statistical analysis was performed with IBM SPSS Statistics 23; continuous variables were analyzed utilizing an independent samples t-test and categorical variables with Pearson’s chi-square test.
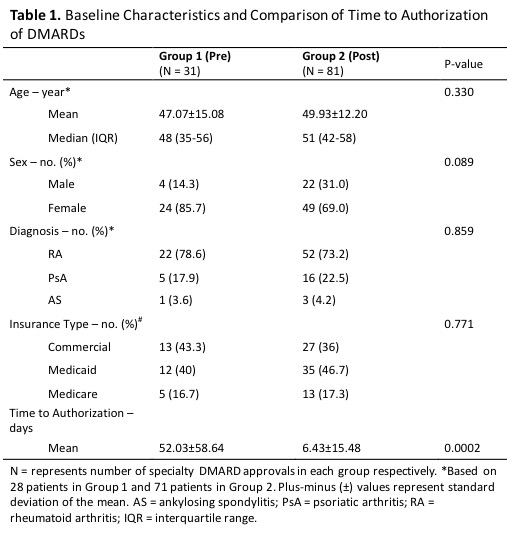
Results: We screened 423 specialty prescriptions; 112 were eligible for study inclusion. Thirty-one specialty prescriptions from 28 patients were completed pre-pharmacist (Group 1) and 81 specialty prescriptions from 71 patients were completed post-pharmacist (Group 2) (Table 1). There were no significant differences between the groups in baseline characteristics that were tested including age, gender, diagnosis, and type of insurance. The majority of patients were women with RA; most patients had commercial insurance or Medicaid. The mean time to authorization was significantly shorter post-pharmacist compared to pre-pharmacist routine care (6.43±15.48 vs. 52.03±58.64 days, p=0.002).
Conclusion: In a large tertiary academic rheumatology practice, the mean time to authorization of specialty DMARDs was significantly reduced to <7 days with establishment of a clinical pharmacist in a collaborative clinical team, facilitating implementation of T2T strategy.
To cite this abstract in AMA style:
Ramey W, Lohr KM, Zeltner M, Herrell Postonl H, Johannemann A, Schadler AD, Lenert A. Biological and Targeted Synthetic Dmards’ Prior Authorization Time Is Significantly Reduced with Pharmacy Presence in the Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/biological-and-targeted-synthetic-dmards-prior-authorization-time-is-significantly-reduced-with-pharmacy-presence-in-the-rheumatology-clinic/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biological-and-targeted-synthetic-dmards-prior-authorization-time-is-significantly-reduced-with-pharmacy-presence-in-the-rheumatology-clinic/