Session Information
Date: Sunday, November 5, 2017
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment I: Novel and Current Therapies
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Low disease activity (LDA) in lupus patients is increasingly a goal of treatment. For instance, Lupus Low Disease Activity State (LLDAS) attainment is associated with reduced damage accrual in patients with SLE (Franklyn et al Ann Rheum Dis 2016). Atacicept targets the B-cell stimulating factors, BLyS and APRIL, and was associated with reduction of disease activity in SLE patients (pts) starting with high disease activity (HDA; SLEDAI-2K ≥10 at Screening) in the Phase IIb ADDRESS II study. We present a post-hoc analysis of LDA attainment by ADDRESS II pts who started with HDA.
Methods: In ADDRESS II (NCT01972568), pts received weekly atacicept (75 or 150 mg SC injection) or placebo (PBO) for 24 weeks (1:1:1 randomization). 3 LDA definitions were used: LDA-1 (SLEDAI-2K ≤ 2); LDA-2 (SLEDAI-2K ≤ 2 and prednisone-equivalent ≤7.5 mg/day); and LLDAS (SLEDAI–2K ≤ 4 without major organ activity, no new disease activity vs previous visit, Physician’s Global Assessment ≤1, prednisone-equivalent ≤7.5 mg/day, and stable maintenance doses of immunosuppressants). Each LDA definition was assessed at each post-baseline study visit. The association of LDA attainment with SLE responder index (SRI)-6 was explored using descriptive statistics, and differences in LDA attainment between HDA pts treated with atacicept vs PBO at Week 24 were analyzed using odds ratio (OR) of LDA attainment estimated from logistic regression.
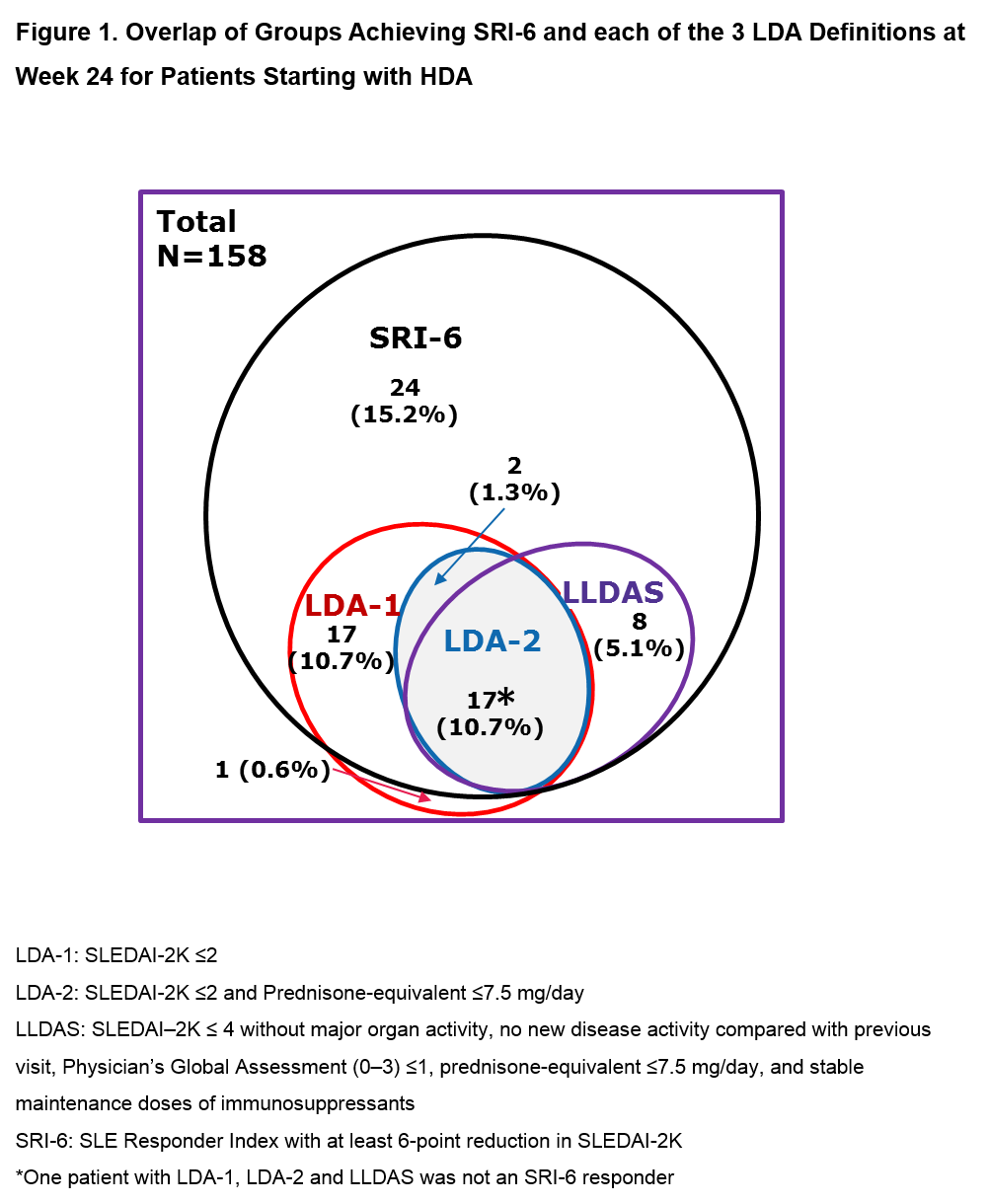
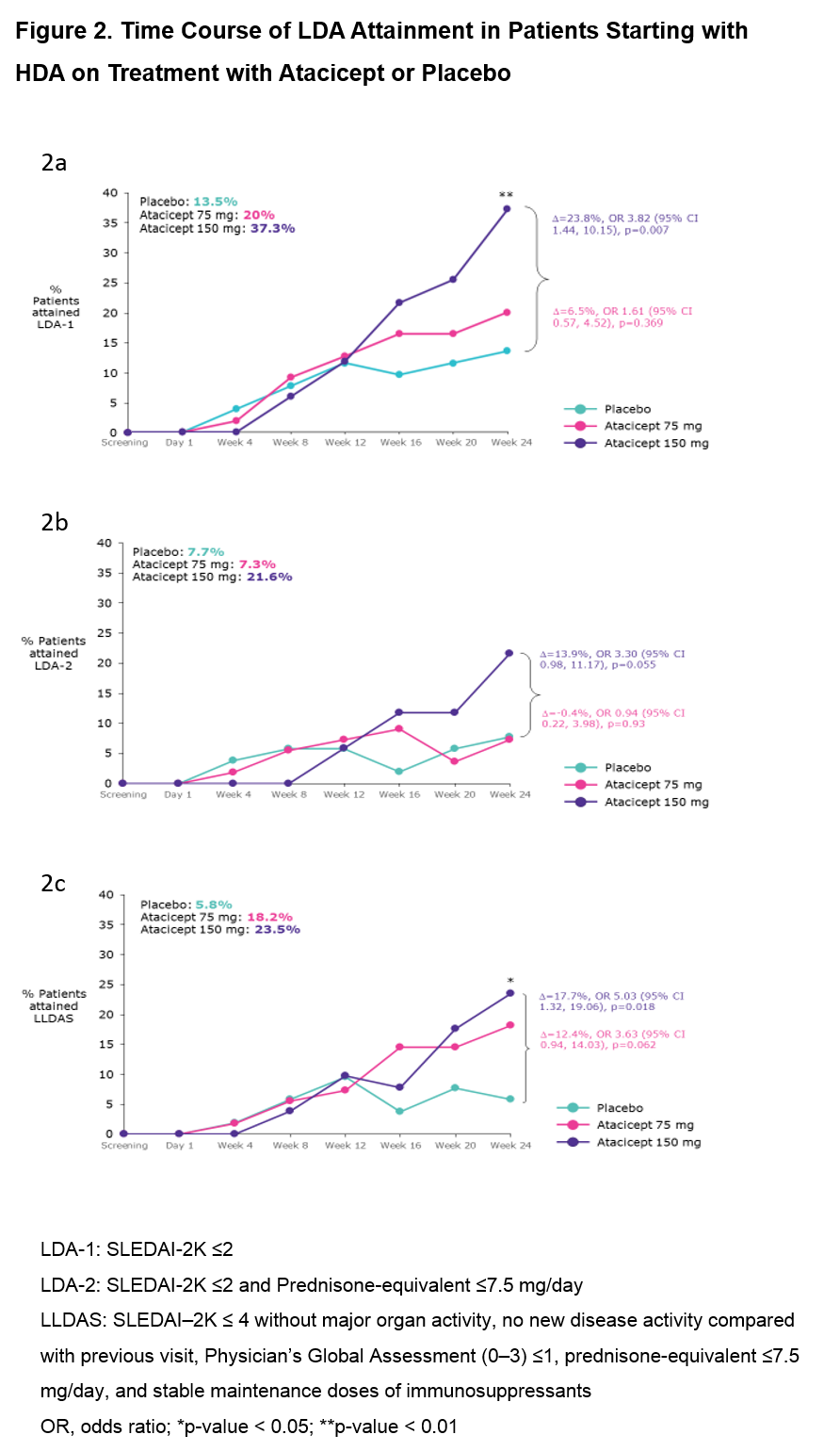
Results: Of the 306 pts in ADDRESS II, 158 (52%) had HDA at Screening (52 PBO; 55 atacicept 75 mg; 51 atacicept 150 mg). Fig 1 shows the attainment of LDA and SRI-6 response at Week 24: 37 pts (23.4%) achieved LDA-1, 19 (12.0%) LDA-2, 25 (15.8%) LLDAS and 67 (42.4%) SRI-6 response, irrespective of treatment. Each LDA definition identified a similar subset of pts, with LDA-2 the most stringent. Of the SRI-6 responders, 64.2% also attained LDA; all except 2 pts who attained LDA were SRI-6 responders. LDA attainment increased with atacicept dose. Pts who received atacicept 150 mg were more likely to attain LDA at Week 24 vs those who received PBO (LDA-1: OR 3.82 [95% CI 1.44, 10.15], p=0.007; LDA-2: OR 3.30 [95% CI 0.98, 11.17], p=0.055; LLDAS: OR 5.03 [95% CI 1.32, 19.06], p=0.018; Fig 2).
Conclusion: For pts in the ADDRESS II study starting with high disease activity, LDA attainment was associated with SRI-6 response. Treatment with atacicept 150 mg vs PBO was associated with a 3- to 5-fold relative increase in the odds of LDA attainment. These results warrant further evaluation of atacicept in SLE, and suggest that LDA endpoints, including LLDAS, which has been validated against damage accrual, can be robust outcome measures in future SLE clinical trials.
To cite this abstract in AMA style:
Morand EF, Merrill JT, Kao AH, Vazquez-Mateo C, Wax S, Chang P, Pudota K, Isenberg DA. Attainment of Low Disease Activity By Patients with Systemic Lupus Erythematosus Starting with High Disease Activity in a 24-Week, Randomized, Placebo-Controlled, Phase IIb Study of Atacicept (ADDRESS II) [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/attainment-of-low-disease-activity-by-patients-with-systemic-lupus-erythematosus-starting-with-high-disease-activity-in-a-24-week-randomized-placebo-controlled-phase-iib-study-of-atacicept-address/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/attainment-of-low-disease-activity-by-patients-with-systemic-lupus-erythematosus-starting-with-high-disease-activity-in-a-24-week-randomized-placebo-controlled-phase-iib-study-of-atacicept-address/