Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Assessment of pain improvement during treatment for rheumatoid arthritis (RA) may help frame patient expectations and may be useful to clinical decision-making and discussions between providers and their patients. Baricitinib (BARI) 4 mg once daily was associated with significant clinical improvements in the Phase 3 study, RA-BEAM, in active RA patients with an inadequate response to methotrexate (MTX) compared with adalimumab (ADA) and placebo (PBO).1 The objective of this post hoc analysis was to evaluate the time and likelihood of achieving different levels of pain control with BARI relative to ADA and PBO.
Methods:
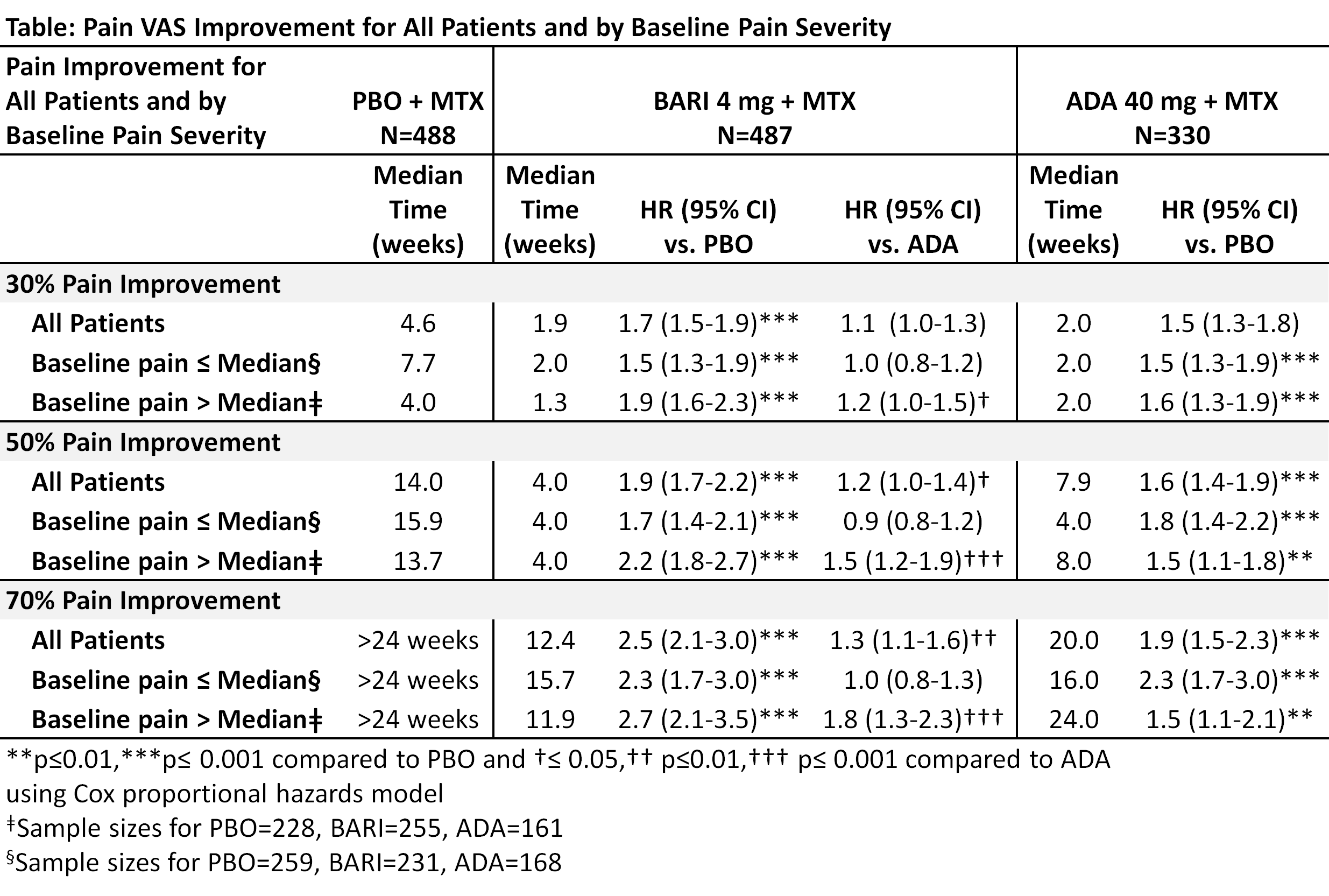
1305 patients on stable background MTX were randomized 3:3:2 to PBO, BARI 4 mg, or ADA 40 mg. The patient’s assessment of pain was assessed with a 0-100 mm visual analog scale (VAS) at each study visit. The likelihood of achieving ≥30%, ≥50%, and ≥70% pain VAS improvement through Week 24 and the median time when 50% of patients achieved these pain improvement thresholds were assessed with Cox proportional hazards models and the cumulative incidence estimate.2 Pain improvement was analyzed by baseline pain VAS subgroup (≤median, >median). Analyses were not adjusted for multiplicity.
Results:
BARI-treated patients were more likely to achieve at least 30%, 50%, and 70% pain improvement than PBO and ADA with HR of 1.7, 1.9, and 2.5, respectively (p<0.001) compared to PBO, and 1.1 (p=0.145), 1.2 (p=0.032), and 1.3 (p=0.003) compared to ADA. The median time for 50% of patients to achieve at least 30%, 50%, and 70% pain improvement, respectively, was 1.9,4.0, and 12.4 weeks for BARI, 2.0,7.9, and 20.0 weeks for ADA, and 4.6, 14.0, and >24 weeks for PBO (Table). The cumulative incidence for achieving 50% pain improvement is presented (Figure). The effects of BARI on pain improvement were consistent regardless of baseline pain severity. In contrast, pain improvement for patients treated with ADA or PBO varied by baseline pain severity (Table).
Conclusion:
BARI demonstrated faster and greater pain improvement than ADA or PBO through Week 24. In addition, unlike ADA and PBO, BARI showed consistent improvement regardless of baseline pain severity.
References:
1Taylor et al. New Engl J Med 2017;376:652-62
2Gooley TA Statist Med 1999
To cite this abstract in AMA style:
Taylor PC, Fleischmann R, Perkins E, Lisse J, Zhu B, Gaich CL, Zhang X, Schlichting DE, Dickson CL, Takeuchi T. Rapid and Sustained Pain Improvement in Rheumatoid Arthritis Patients Treated with Baricitinib Compared to Adalimumab or Placebo [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/rapid-and-sustained-pain-improvement-in-rheumatoid-arthritis-patients-treated-with-baricitinib-compared-to-adalimumab-or-placebo/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-and-sustained-pain-improvement-in-rheumatoid-arthritis-patients-treated-with-baricitinib-compared-to-adalimumab-or-placebo/