Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
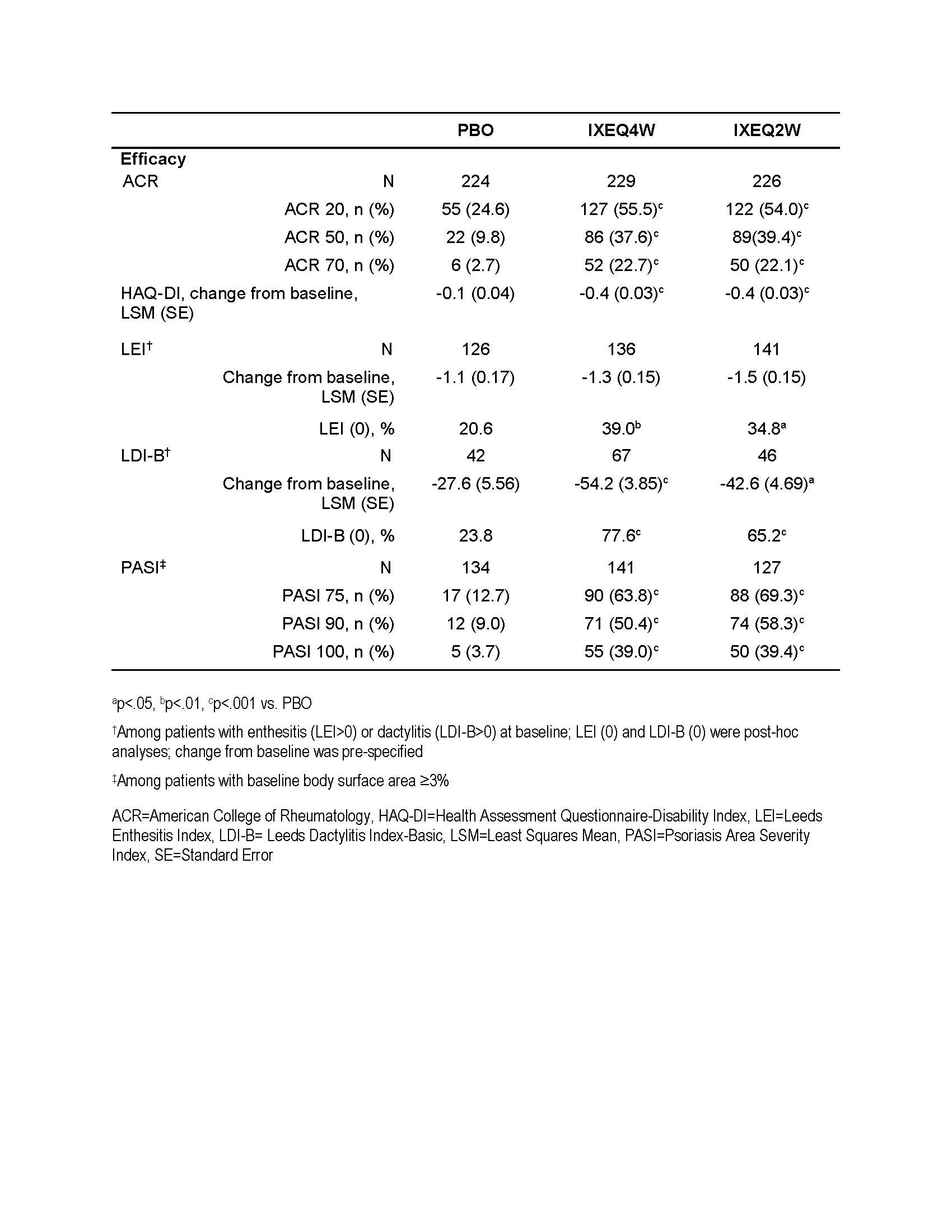
Background/Purpose: Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets interleukin-17A. Here, we present integrated efficacy data at Week 24 from two phase 3 trials of IXE for the treatment of psoriatic arthritis (PsA).
Methods: Patients with active PsA (SPIRIT-P1) and with prior lack of efficacy or intolerance to TNF-inhibitor(s) (SPIRIT-P2) were randomized to placebo (PBO, N=224), 80 mg IXE every 4 weeks (IXEQ4W, N=229) or every 2 weeks (IXEQ2W, N=226), after a 160 mg starting dose. All patients considered as inadequate responders at Week 16 received rescue therapy (changes in background therapy), while inadequate responders to PBO were also re-randomized to IXEQ4W or IXEQ2W. Continuous data were analyzed using mixed-effects model for repeated measures; categorical data, using a logistic regression model with missing values imputed by non-responder imputation.
Results: At Week 24, significantly more patients treated with either dose of IXE (p<.001) compared to PBO achieved the primary endpoint of ACR 20, as well as ACR 50, ACR 70, and HAQ-DI change from baseline. Treatment with either dose of IXE resulted in significantly more patients achieving resolution of enthesitis (LEI; p<.05) and dactylitis (LDI-B; p<.001) compared to PBO. LDI-B improvements from baseline were also significantly greater (p<.05) for IXE-treated patients versus PBO. Finally, greater skin clearance (via PASI improvement) was significantly higher for IXE-treated patients (p<.001).
Conclusion: Patients treated with either dose regimen of IXE achieved significantly greater improvements in arthritis, physical function, and skin lesions compared to PBO at Week 24.
To cite this abstract in AMA style:
Combe B, Nash P, Adams D, Kerr L, Benichou O. Integrated Efficacy Results from Two Phase 3 Trials of Ixekizumab for the Treatment of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/integrated-efficacy-results-from-two-phase-3-trials-of-ixekizumab-for-the-treatment-of-psoriatic-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-efficacy-results-from-two-phase-3-trials-of-ixekizumab-for-the-treatment-of-psoriatic-arthritis/