Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Many subjects (sbj) with chronic plaque psoriasis exhibit nail and scalp involvement that can markedly affect quality of life and be difficult to treat. The phase 3b LIBERATE study evaluated efficacy and safety of apremilast or etanercept vs. placebo in biologic-naive sbj with moderate to severe plaque psoriasis. Efficacy assessments included effects on preexisting nail and scalp disease and skin lesions.
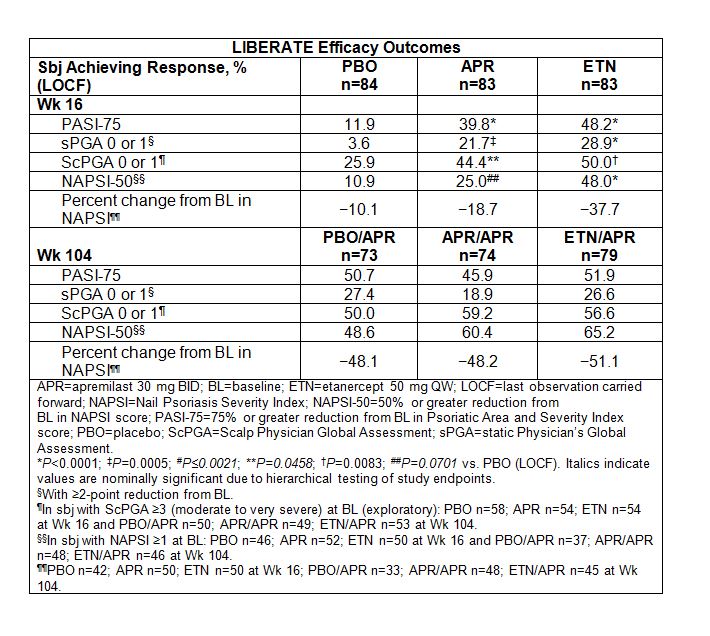
Methods: In this double-blind, double-dummy study, sbj were randomized (1:1:1) to placebo (PBO), apremilast 30 mg BID (APR), or etanercept 50 mg QW (ETN) through Wk 16; thereafter, all sbj switched to or continued APR (PBO/APR, ETN/APR, APR/APR) through Wk 104. The primary endpoint was achievement of a PASI-75 response at Wk 16 with APR vs. PBO; the secondary endpoint was PASI-75 achievement at Wk 16 with ETN vs. PBO. Physician assessments were conducted for overall psoriatic activity (static Physician’s Global Assessment [sPGA]); scalp disease activity (Scalp Physician Global Assessment [ScPGA], limited to sbj with score ≥3 at baseline [BL], indicating moderate to very severe scalp disease); and nail disease (Nail Psoriasis Severity Index [NAPSI], limited to sbj with active disease [NAPSI ≥1] in the target nail at BL). Responses were assessed through Wk 104 using last-observation-carried-forward methodology.
Results: The APR extension phase (Wks 16 to 104) included 226 sbj (PBO/APR n=73; APR/APR n=74; ETN/APR n=79). At Wk 16, PASI-75 response vs. PBO was significant for both APR and ETN; long-term treatment with APR maintained both PASI-75 and sPGA 0 or 1 response levels (Table). Improvements were seen in nail and scalp disease at Wk 16, and the proportion of responders continued to increase with APR treatment over 104 wks and in sbj who switched from ETN to APR (Table). ScPGA 0 or 1 was achieved by 50.0% to 59.2% of sbjsacross treatment arms, and mean percent improvement from BL NAPSI score ranged from −48.1% to −51.1% (Table); the proportion of sbj achieving NAPSI-50 response ranged from 48.6% to 65.2%. Adverse events (AEs) occurring in ≥5% of sbj during Wks 0 to 16 were diarrhea, nausea, nasopharyngitis, upper respiratory tract infection, headache, and tension headache; long-term assessment by exposure-adjusted incidence rates (EAIR)/100 sbj-yrs showed no increase with longer-term APR exposure. No increase in EAIR/100 sbj-yrs of serious AEs occurred during the APR extension phase (4.01 to 5.49, across groups) vs. Wks 0 to 16 (PBO 0.0; APR 12.57; ETN 7.91). Changes in laboratory parameters were infrequent and transient; EAIR/100 sbj-yrs remained low across groups through 104 wks.
Conclusion: APR demonstrated efficacy through Wk 104 in sbj who continued APR and sbj who switched from PBO or ETN to APR at Wk 16. The AE profile remained consistent with prolonged APR exposure; no new safety or tolerability issues were observed through Wk 104 in sbj with moderate to severe plaque psoriasis.
To cite this abstract in AMA style:
Reich K, Goodfield M, Green L, Nograles K, Chen R, Levi E, Langley RGB. Efficacy and Safety of Apremilast through 104 Weeks in Subjects with Moderate to Severe Psoriasis Randomized to Placebo, Apremilast, or Etanercept Who Continued on or Switched to Apremilast after Week 16 in a Phase 3b Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-apremilast-through-104-weeks-in-subjects-with-moderate-to-severe-psoriasis-randomized-to-placebo-apremilast-or-etanercept-who-continued-on-or-switched-to-apremilast-after-week/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-apremilast-through-104-weeks-in-subjects-with-moderate-to-severe-psoriasis-randomized-to-placebo-apremilast-or-etanercept-who-continued-on-or-switched-to-apremilast-after-week/