Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The goal of therapy of rheumatoid arthritis (RA) is to achieve a state of low disease activity (LDA) or remission and reduce joint damage and disability. When treatment with one agent is unsuccessful, an alternative DMARD, biologic or small molecule drug will be prescribed. This retrospective study reports the frequency and reasons for drug changes in RA patients in a tertiary US academic practice.
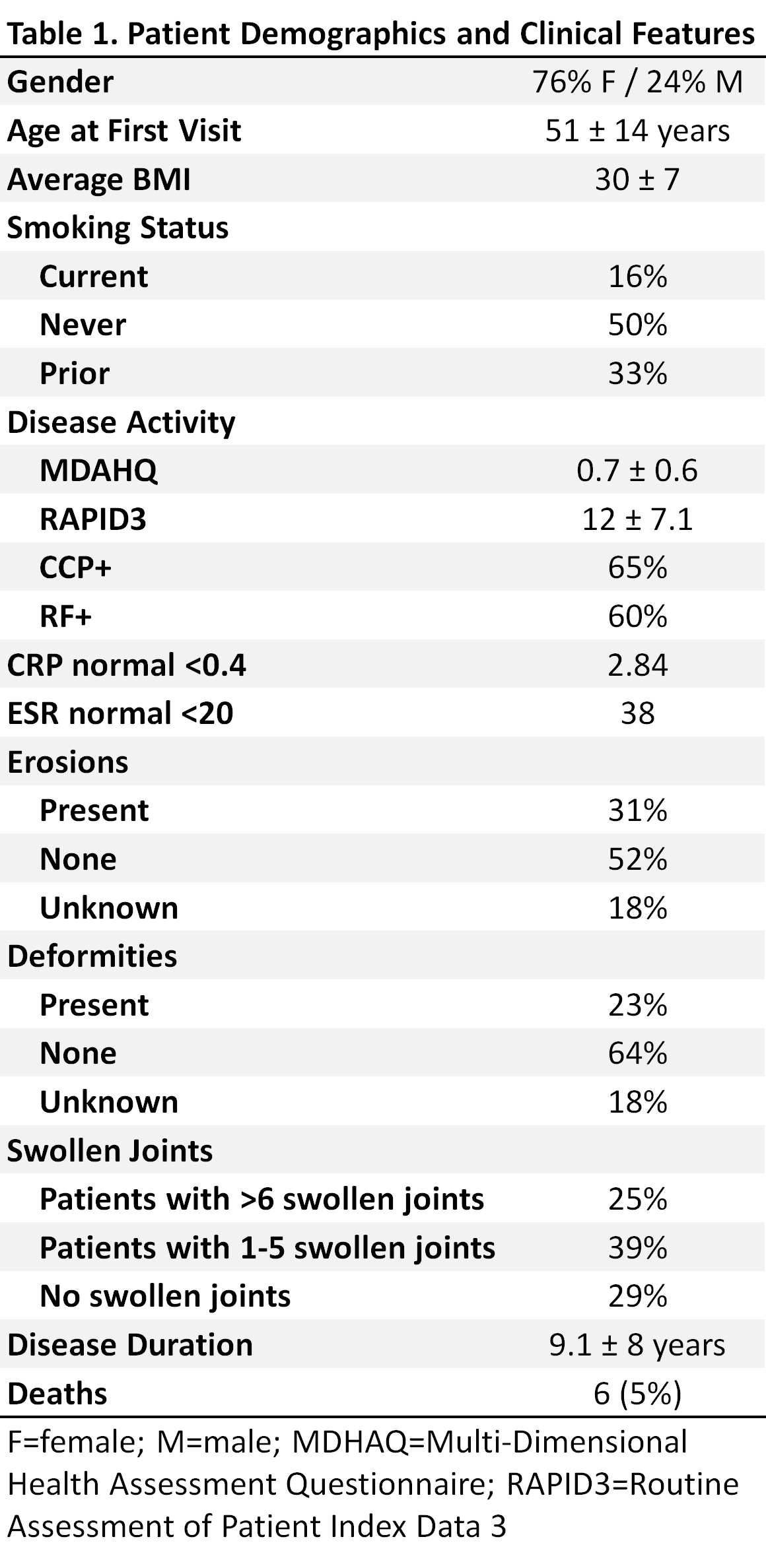
Methods: Records of 1300 RA patients were reviewed by 3 rheumatologists to select patients who received at least one biologic (b) DAMRD, had no other rheumatic disease, no interstitial lung disease or chronic infections, and were followed for at least one year to be included in this report. The study cohort consists of 198 patients who fulfilled the inclusion criteria. Patient demographics, drugs used, duration of use, and reasons for change of therapy were recorded.
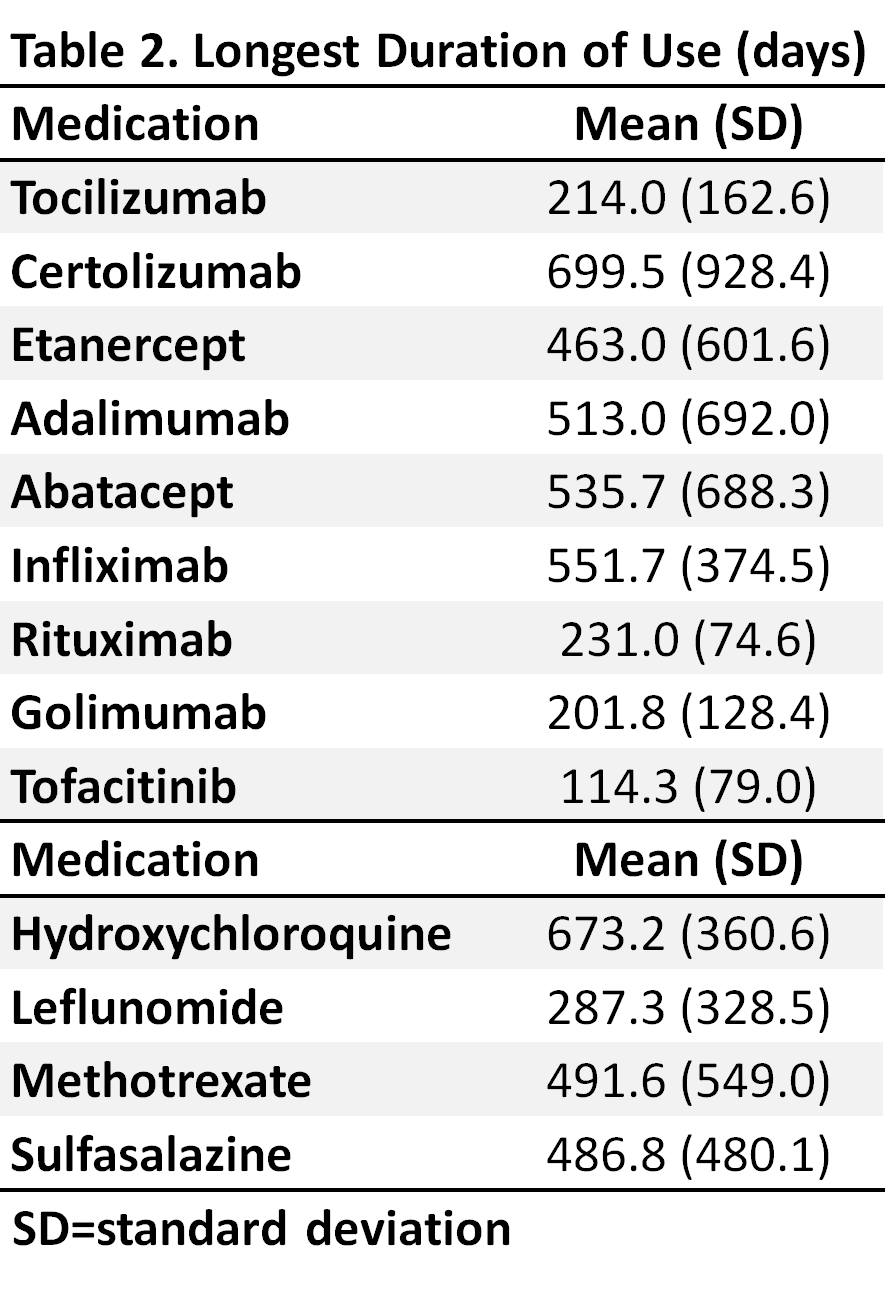
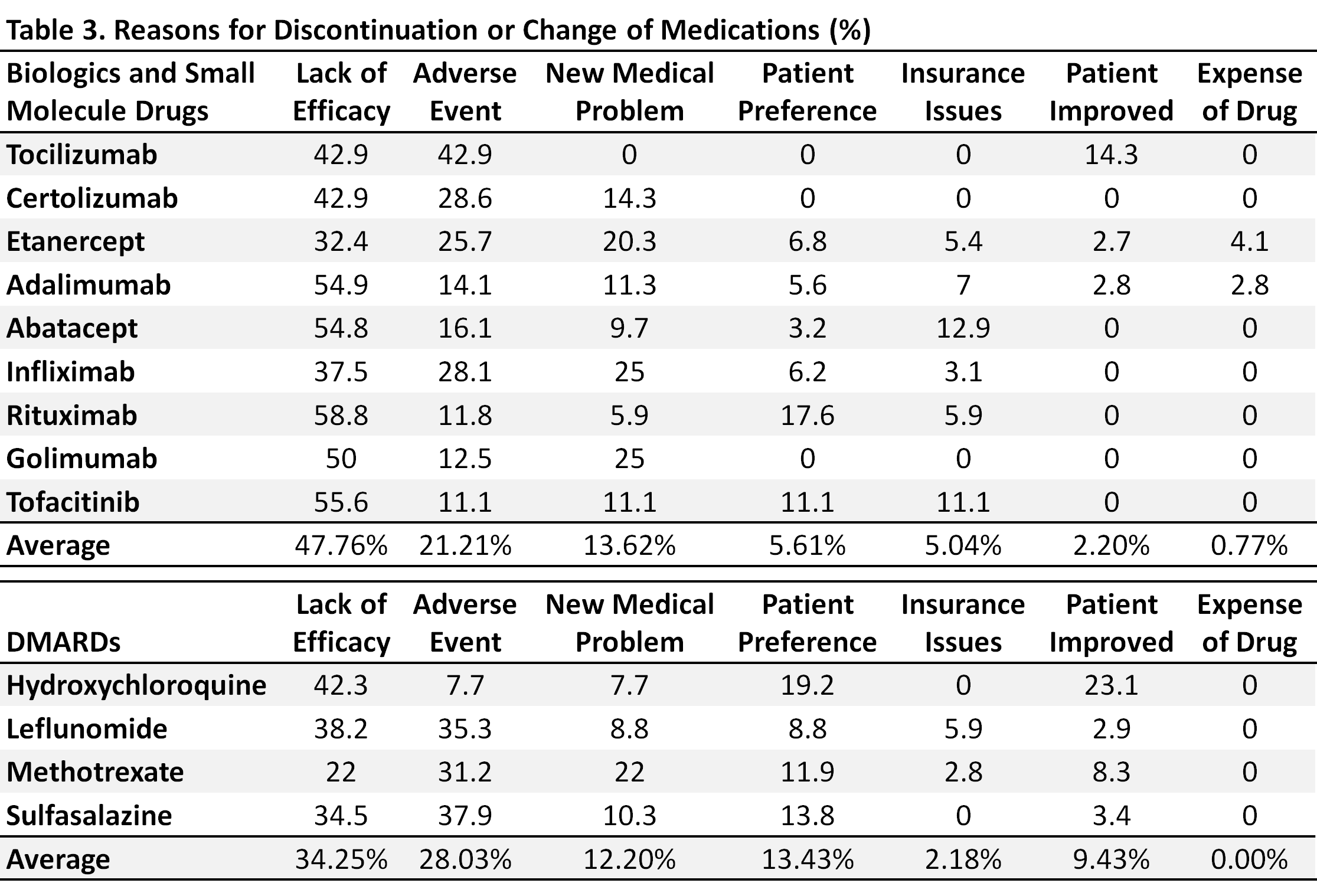
Results: Of the 198 patients, 75% were females, average age was 51, 65% were ACPA positive, and 31% of patients had erosions at first visit. Patient demographics are summarized in Table 1. These patients received an average of 2.5 drugs before their first visit. They changed to a new drug an average of 2.4 times during their average follow up visits of 6.2 years. The longest average duration of continuous use for any of the DMARDs was 6-24 months (Table 2). The main reasons for discontinuation of these medications were lack of efficacy and the occurrence of adverse events (AEs). The rate of AEs among patients receiving bDMARDs was similar to that in patients receiving conventional DMARDS. The reasons for discontinuation of specific medications are summarized in Table 3.
Conclusion: This retrospective review suggests that most patients with RA will require a change of therapy every 2-3 years, primarily due to perceived lack of efficacy or the occurrence of AEs. The frequent change of therapy in patients with chronic disease like RA suggests that patients will use many different drugs in the course of their illness. In the absence of an effective biomarker that predicts response to a specific medication, therapeutic agents selected may not be efficacious and require early recognition of lack of efficacy and subsequent change of therapy.
To cite this abstract in AMA style:
Meehan R, Amigues I, Muram D, Hoffman E, Crooks J, Truong T, Zelarney P. Efficacy, Tolerability and Reasons for Changing Dmards, Biologics and Small Molecule Drugs in RA Patients without RA Lung Disease from a United States Tertiary Referral Center [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-tolerability-and-reasons-for-changing-dmards-biologics-and-small-molecule-drugs-in-ra-patients-without-ra-lung-disease-from-a-united-states-tertiary-referral-center/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-tolerability-and-reasons-for-changing-dmards-biologics-and-small-molecule-drugs-in-ra-patients-without-ra-lung-disease-from-a-united-states-tertiary-referral-center/