Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
To investigate the efficacy and safety of zoster vaccines administered in rheumatoid arthritis(RA) patients taking methotrexate(MTX).
Methods:
By reviewing data through the Kaiser Permanente electronic medical record (EMR) retrospectively, we identified 893 adult RA patients who received zoster vaccination between January 1, 2007 and December 31, 2012 and had at least 2 RA diagnoses documented within one year prior to the vaccination date. Patients treated with any biologic DMARDs within 6 months prior to the index dates were excluded. The cohort was followed until the occurrence of zoster infection, disenrollment, death or the end of study, whichever came first. The primary outcome, vaccination safety, was measured by time to occurrence of zoster infections within 42 days after zoster vaccines administered. The secondary outcome, vaccine efficacy, measured by time to occurrence of zoster infections (after day 42) until the end of follow-up period, was studied among those who maintain the treatment during follow-up period (n=762). Dosage effect was also analyzed among those taking MTX (n=342) by comparing patients taking >=25mg/week and those taking 22.5mg/week or less. Cox proportional model was used to analyze hazard ratios between groups.
Results:
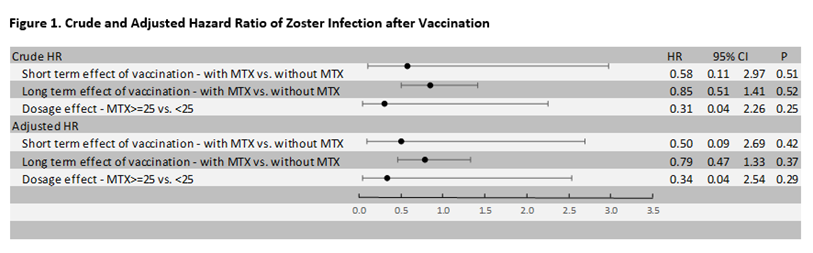
Among 893 RA patients who were given zoster vaccines, 366 patients were taking MTX within 6 months prior to the zoster vaccination and 527 patients were not taking MTX. At baseline, 707(79%) patients were female with a mean age of 70.1 (SD: .5) and disease duration of 6.6 (3.9) years. The analysis showed that the rate of zoster infection was 0.13 per 1000-person day in the MTX group and the rate was 0.23 per 1000-person day in the non-MTX group. We found no significant statistical difference in the rate of zoster infections within the first 42 days of vaccination with adjusted hazard ratio of .50(95% CI 0.09-2.69, p-value=0.42). During the subsequent follow-up period, 742 patients continued their baseline treatment. The rate of zoster infection was 15.9 per 1000-person year in the MTX group and the rate was 18.6 per 1000-person year in the non-MTX group with the adjusted hazard ratio 0.79(95% CI 0.47-1.33, p-value=0.37). Among the 334 patients taking MTX, 292 patients were taking a dose of 22.5 mg or under while 42 were taking the dose of 25mg or more. The final analysis from the model showed no significant difference in dosage effect, the adjusted hazard ratio was 0.34(95% CI 0.04-2.54, p-value=0.29).
Conclusion:
Zoster vaccination is safe to be given to RA patients taking MTX regardless of the dosage. The vaccination is equally effective in RA patients taking MTX and not taking MTX.
To cite this abstract in AMA style:
Lin A, Li Q, Shi J, Lin S, Wang D, Wan J, Lee K, Tseng HF, Cheetham T. The Occurrence of Shingles and the Effect of Zoster Vaccination with the Use of Methotrexate in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-occurrence-of-shingles-and-the-effect-of-zoster-vaccination-with-the-use-of-methotrexate-in-rheumatoid-arthritis-patients/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-occurrence-of-shingles-and-the-effect-of-zoster-vaccination-with-the-use-of-methotrexate-in-rheumatoid-arthritis-patients/