Session Information
Date: Sunday, November 5, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster I: Treatment Patterns and Response
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Dose reduction and discontinuation of TNF inhibitors (TNFi) have been shown feasible in a large proportion of RA patients with low disease activity.1 To date, no predictors for successful dose reduction or discontinuation have been identified.2 Calprotectin (a heterodimer of S100A8/S100A9) might be a promising biomarker in this context.3,4
Objectives: To investigate the predictive value of baseline serum calprotectin for successful TNFi dose reduction or discontinuation in RA patients with low disease activity.
Methods: Data was derived from the intervention arm of the DRESS (Dose REduction Strategies of Subcutaneous TNFi) study, which showed non-inferiority of a dose reduction strategy of adalimumab or etanercept compared to usual care.1 TNFi dose interval was reduced stepwise every 3 months until flare (DAS28-CRP increase >1.2 or >0.6 if current DAS28-CRP ≥3.2) or discontinuation. Patients were classified at 18 months as being successfully dose reduced, discontinued or not able to reduce the TNFi dose. At baseline, quantification of calprotectin was carried out on serum samples using ELISA. Calprotectin levels were compared between each group and receiver-operator-characteristic (ROC) curves were created. In addition, calprotectin was correlated cross-sectionally with several clinical markers for disease activity.
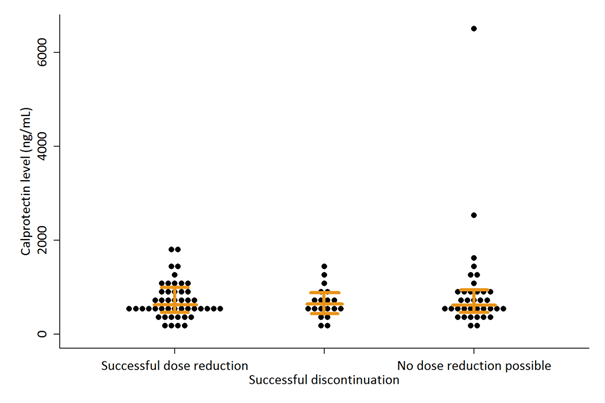
Results: Calprotectin levels were available from 102 of 121 patients randomised to the intervention group; 61% were women, 63% received etanercept and 37% received adalimumab. Overall, 46% of patients successfully reduced the TNFi dose, 19% of patients successfully discontinued TNFi and 35% of patients could not reduce the TNFi dose. In these groups, median calprotectin levels were 599 ng/ml (p25-p75: 473-965), 629 ng/mL (p25-p75: 453-896) and 624 ng/mL (p25-p75: 514-931) (p=0.801) (Figure 1). The area under the ROC-curve was 0.52 (95% CI: 0.40-0.63) for predicting successful TNFi dose reduction, 0.53 (95% CI: 0.38-0.67) for successful TNFi discontinuation and 0.54 (95% CI: 0.42-0.66) for no dose reduction possible. Calprotectin levels were weakly correlated with C-reactive protein (CRP) levels with a Spearman ρ of 0.21 (p=0.03). No significant correlation was found between calprotectin and age, gender, DAS28-CRP, rheumatoid factor or ACPA positivity.
Conclusion: Serum calprotectin is not predictive for successful TNFi dose reduction or discontinuation in RA patients with low disease activity, and calprotectin was only weakly correlated to CRP levels. These results might be caused by the lack of variability in calprotectin levels at baseline as all patients were in low disease activity state.
Figure 1 Calprotectin levels per outcome
References:
1 van Herwaarden et al. BMJ 2015;350:h1389
2 Tweehuysen et al. Arthritis Rheumatol 2017;69(2):301-308
3 Hammer et al. Ann Rheum Dis 2010;69(1):150-4
4 Choi et al. Ann Rheum Dis 2015;74(3):499-505
To cite this abstract in AMA style:
den Broeder N, Tweehuysen L, van Herwaarden N, Vogl T, van den Hoogen FHJ, Thurlings R, Den Broeder AA. Serum Calprotectin Is Not Predictive for Successful Dose Reduction or Discontinuation of TNF Inhibitors in RA Patients with Low Disease Activity [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/serum-calprotectin-is-not-predictive-for-successful-dose-reduction-or-discontinuation-of-tnf-inhibitors-in-ra-patients-with-low-disease-activity/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-calprotectin-is-not-predictive-for-successful-dose-reduction-or-discontinuation-of-tnf-inhibitors-in-ra-patients-with-low-disease-activity/