Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Canakinumab (CAN) has demonstrated its efficacy and safety in systemic juvenile idiopathic arthritis in clinical trials. We report on the experience with CAN in the clinical practice.
Methods: Surveillance of patients exposed to biologics is performed by the BIKER registry. Data on patients’ and disease characteristics, disease activity and safety reports until Dec. 2016 were analysed.
Results: Until June 2017, 39 JIA patients were registered in the German BIKER registry in whom CAN was started, representing 59.2 PY of exposure. In 14 patients CAN was used as first biologic agent. 25 patients were pretreated with other biologics, 14 received 1, 7 two, 3 three and 1 four biologics. 15 had been treated with Tocilizumab, 11 with Anakinra, 9 with Etanercept and 9 with Adalimumab. Of interest, 3 patients in the pre-exposed cohort had experienced a macrophage activation syndrome. Patient’s and disease characteristics comparison of biologics naïve and preexposed patients are given below.
Patients pretreated were older, had a longer disease duration and more comorbidities (Macrophage activation syndrome, pericarditis, pleuritic organomegaly and Cushing’s) than naïve patients. The proportion of patients with active arthritis, active systemic features and both were comparable.
Disease activity at baseline (number of active joints, Patient’s and physicians’ global, ESR, CRP and the JADAS) was higher in the biologic naïve cohort suggesting some clinical benefit from pretreatment in the exposed cohort. Dosing of CAN was comparable (3.9+/-0.4 vs. 3.5+/-0.7 mg/kg) as well as the median treatment duration.
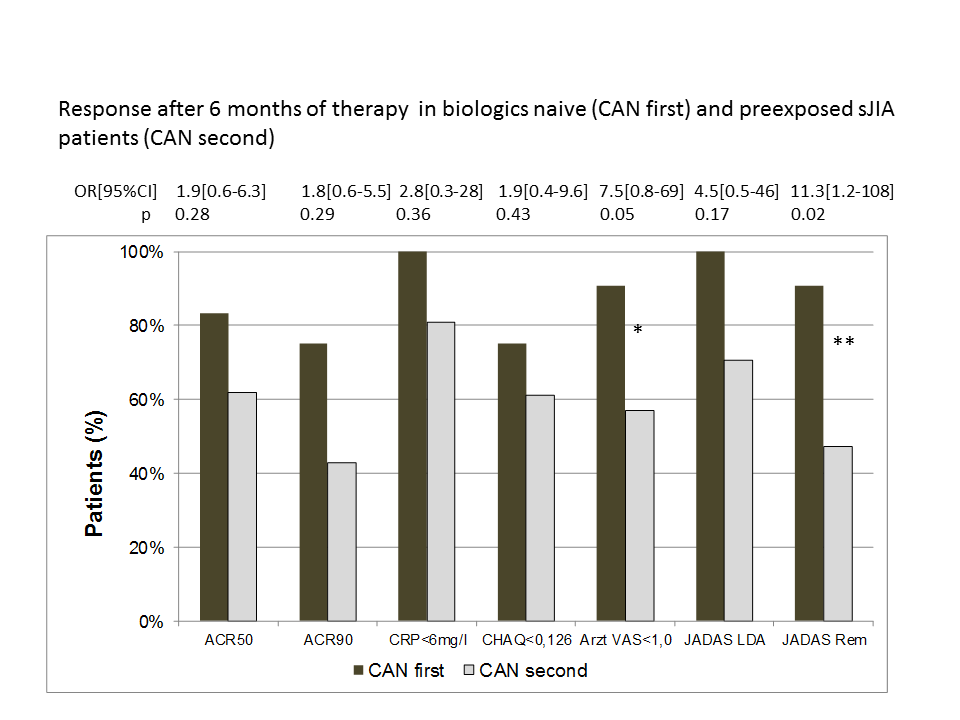
Treatment efficacy at 6 month of treatment was stronger in the naïve cohort with more patients reaching a PedACR50/90 response, CRP normalisation, normal CHAQ, physician global indicating no activity (p=0.05) JADAS remission (p=0.02). Treatment with CAN was discontinued by 42% in the naïve cohort and 48% in the exposed cohort. Reasons for withdrawals were inefficacy (n=7; 19%), intolerance (n=2; 5%) and remission (n=7; 19%) of the disease and other (n=2;5%).
Conclusion: First experience with CAN for treatment of systemic JIA in clinical practice is presented. A high proportion of patients gained significant response to treatment. JADAS remission was reached in significantly more biologics naïve patients while few patients discontinued treatment in remission so far. Intolerance was rare. The further long term surveillance of patients exposed to biologics is intended by the registry.
Table 1: Baseline characteristics in the comparison groups
|
Biologics naive |
Biologics pre-exposed |
|
|
N ( female gender) |
14 (28%) |
25 (52%) |
|
Age at JIA onset (years); Median (IQR)
|
3.3 (2.7-5.2) |
3.7 (2.6-7.1) |
|
Disease duration (years); Median (IQR)
|
0.7 (0.2-5.6) |
1.9 (0.6-8.7) |
|
Concomitant treatment at baseline: NSAIDS
|
7 (50%) |
10 (40%) |
|
Steroids |
6 43%) |
11 (44%) |
|
MTX |
3 (21%) |
6 (24%) |
|
Patients with active joints |
8 (57%) |
10 (40%) |
|
Patients with active systemic features
|
9 (64%) |
10 (40%) |
|
Active joint count; Median (IQR)
|
2.5 (0-3) |
0 (0-3) |
|
Physician global VAS (0-10); Median (IQR)
|
5.2+/-2.8; 6.2 (3.2-7.2) |
3.8+/-3m.4 ; 3.7 (0.7-6.3) |
|
Patient Global VAS (0-10); Median (IQR) |
4.8+/-2.9; 4.6 (2.7-7) |
3.3+/-2.9; 2.6 (0.6-5.1) |
|
CHAQ-DI (0-3); Median (IQR) |
0.5 (0.38-1.24) |
0.65+/-0.84; 0.25 (0-1.22) |
|
ESR (mm/h); Median (IQR)
|
28 (10-55) |
9 (4-18.5) |
|
CRP (mg/l); Median (IQR)
|
43 (25-109) |
3 (1-11) |
|
JADAS10; Median (IQR) |
15.2 (14-20.9) |
9.5(5.5-11.8) |
To cite this abstract in AMA style:
Horneff G, Foeldvari I, Tenbrock K, Minden K, Kuemmerle-Deschner JB. Canakinumab First or Second Choice in Systemic Juvenile Idiopathic Arthritis – Experience from Clinical Practice [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/canakinumab-first-or-second-choice-in-systemic-juvenile-idiopathic-arthritis-experience-from-clinical-practice/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/canakinumab-first-or-second-choice-in-systemic-juvenile-idiopathic-arthritis-experience-from-clinical-practice/