Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Sirukumab is a human monoclonal antibody that selectively binds to the IL-6 cytokine with high affinity and is under development for rheumatoid arthritis (RA) and other diseases. This Phase 3 global study (SIRROUND-T) evaluated the efficacy and safety of sirukumab in patients (pts) with active RA who were refractory or intolerant to anti- TNF agents.
Methods: Eligible pts were ≥18 years, had moderate to severe active RA, and had documented lack of benefit to ≥1 anti-TNF agent or intolerance to ≥2 anti-TNF agents. Pts were randomized 1:1:1 to sirukumab SC 50mg q4w, sirukumab SC 100mg q2w, or placebo SC q2w. Pts randomized to placebo at Wk 0 who had <20% improvement in tender/swollen joints at Wk 18 and pts who remained on placebo at Wk 24 were re-randomized to sirukumab through Wk 52. The primary endpoint was the proportion of pts achieving ACR20 response at Wk 16; major secondary endpoints were change from baseline in HAQ-DI score, proportion of pts achieving ACR50 response, and proportion of pts achieving DAS28 (CRP) remission at Wk 24.
Results: 878 pts (placebo, n=294; sirukumab, n=292 per dose group) were randomized and evaluated for efficacy/safety. All pts had taken ≥1 prior anti-TNF, and 39.4% had taken ≥2 prior anti-TNFs. ~39% of pts had previously received biologics other than anti-TNFs. 74.6% of pts were currently taking methotrexate. A significantly higher proportion of pts achieved ACR20 at Wk 16 with sirukumab (50 mg q4w, 40.1%; 100 mg q2w, 45.2%) vs placebo (24.1%); all major secondary endpoints were met for both sirukumab doses (Table; all P<0.001). ACR responses, DAS28 (CRP) remission rates, and HAQ-DI score improvements on sirukumab were maintained through Wk 52. In pts with ≥2 prior biologics (including anti-TNFs; n = 523), both sirukumab 50 mg q4w (42.5%) and 100 mg q2w (42.4%) had greater proportions of ACR20 responders vs placebo (20.9%). Significantly greater improvements were also observed in the physical and mental component summary scores of the SF-36 Health Survey with both sirukumab doses vs placebo at Wk 24 (all P<0.001) and were maintained through Wk 52. For the placebo-controlled phase (up to Wk 24), the incidence of AEs was numerically higher in the sirukumab 100 mg group (68.2%) than in the placebo (61.9%) or sirukumab 50 mg (61.5%) groups. With placebo, sirukumab 50 mg, and sirukumab 100 mg, respectively, the incidence of SAEs was 5.1%, 8.3%, and 7.6%. Up to Wk 52 with sirukumab 50 mg q4w and 100 mgq2w, respectively, incidences of AEs were 79.6% and 81.3% and SAEs were 14.2% and 13.2%.
Conclusion: In this difficult to treat population that was intolerant/refractory to anti-TNFs and other biologics, sirukumab SC 50mg q4w and 100mg q2w reduced RA signs/symptoms, even in pts who had failed ≥2 prior biologics, and improved physical function and patient reported outcomes. The safety profile of sirukumab was similar to the known safety profile of anti–IL-6 receptor treatment. 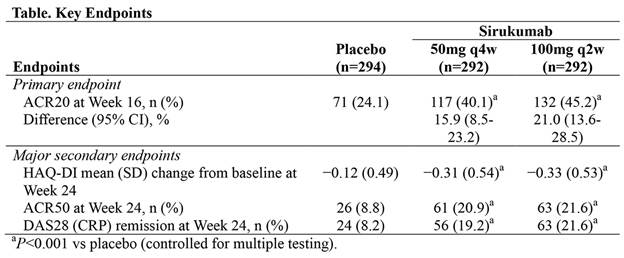
To cite this abstract in AMA style:
Aletaha D, Bingham C III, Tanaka Y, Agarwal P, Kurrasch R, Tak PP, Popik S. Efficacy and Safety of Sirukumab, an Anti–IL-6 Cytokine Monoclonal Antibody, in Patients with Active Rheumatoid Arthritis Despite Anti-TNF Therapy: Results from a Randomized, Double-Blind, Placebo-Controlled, Global, Phase 3 Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-sirukumab-an-anti-il-6-cytokine-monoclonal-antibody-in-patients-with-active-rheumatoid-arthritis-despite-anti-tnf-therapy-results-from-a-randomized-double-blind-pla/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-sirukumab-an-anti-il-6-cytokine-monoclonal-antibody-in-patients-with-active-rheumatoid-arthritis-despite-anti-tnf-therapy-results-from-a-randomized-double-blind-pla/
