Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pt) with Rheumatoid arthritis (RA) who are treated with adalimumab (ADA) are offered a Patient Support Program (PSP) with variety of services. To date, no prospective study has been conducted to analyze the acceptance and the impact of these PSPs on treatment effectiveness and pt satisfaction. The purpose of this study was to examine the effectiveness of ADA on rheumatoid arthritis (RA) treatment course in the context of PSP participation.
Methods: PASSION (NCT01383421) was a 78-week (wk) post-marketing observational study of pts with RA receiving ADA in routine clinical care. Pts from the EU, Israel, Mexico, Puerto Rico, and Australia with an insufficient response to ≥1 disease-modifying antirheumatic drug (DMARD) newly initiating ADA (1 prior biologic DMARD was allowed) were enrolled. The primary endpoint was the % of pts achieving the minimal clinically important difference (MCID; improvement of ≥0.22) in the Health Assessment Questionnaire Disability Index (HAQ-DI) at wk 78 vs baseline (BL). Non-responder imputation (NRI) was used to account for the missing values. Secondary clinical parameters included % of pts achieving MCID in HAQ-DI at wks 24 and 52 vs BL and changes in the 28-joint DAS based on CRP (DAS28(CRP)), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI) at wks 24, 52, and 78 vs BL. Pts were categorized based on their participation in the PSP: ever (PSP users) vs never (PSP non-users) and outcomes were compared after adjusting for corresponding BL values.
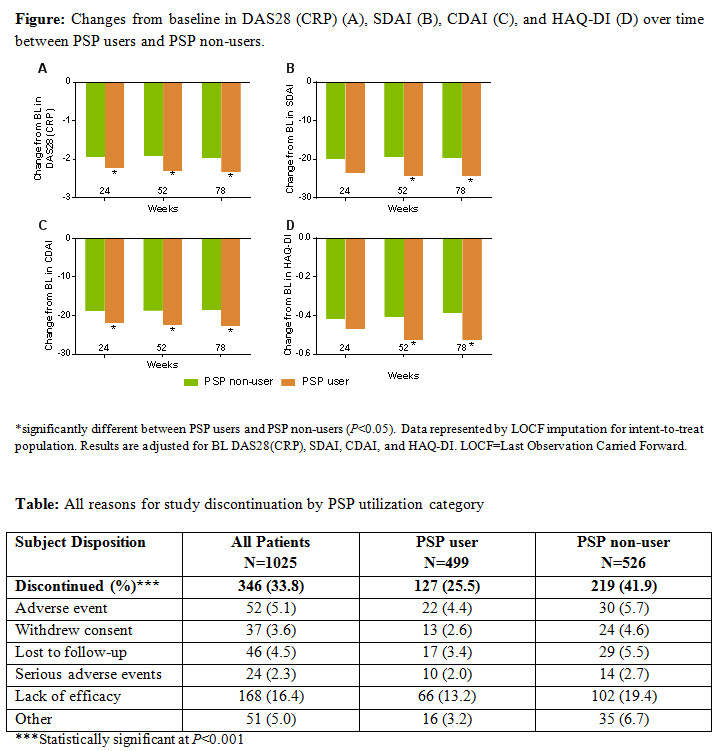
Results: 1,025 pts were included in the Intent-to-treat population (BL: mean age, 54.3 years (y); % female, 77.1%; mean RA duration, 7.8 y; mean HAQ-DI, 1.5; mean DAS28(CRP), 5.3; mean SDAI, 35.6; mean CDAI, 33.3; 17.8% pts had received prior biologic DMARD. Overall, 48.7% pts were PSP users. Approximately 72.9 % (as observed) and 42.8% (NRI) of PSP-users surpassed the MCID in HAQ-DI at wk 78. The percentage of pts achieving MCID in the HAQ-DI was higher in PSP users vs PSP non-users (48.1% vs 37.8%, NRI) at wk 78 (P<0.001). Significant changes (P≤0.05) from BL to wk 78 were observed for pts using the PSP vs PSP non-users in HAQ-DI (0.53 vs 0.39), DAS28(CRP) (−2.33 vs −1.97), SDAI (−24.5 vs −19.8), and CDAI (−22.66 vs −18.55) scores (Figure). Study discontinuation rates were significantly (P<0.001) lower among PSP-users vs PSP non- users (25.5% vs 41.6%). Reasons for discontinuations are listed in the Table.
Conclusion: The final study results showed that, in pts with moderate to severe RA who initiated ADA, significantly better improvement in functional and clinical outcomes was achieved in the PSP users vs the PSP non-users. Improvements were achieved at early timepoints and continued to increase throughout the study.
To cite this abstract in AMA style:
van Den Bosch F, Östör A, Wassenberg S, Anderson JK, Chen N, Wang C, Garg V, Kalabic J. Impact of Participation in the Adalimumab (Humira) Patient Support Program on Functional and Clinical Outcomes Among Patients with Rheumatoid Arthritis: Passion Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/impact-of-participation-in-the-adalimumab-humira-patient-support-program-on-functional-and-clinical-outcomes-among-patients-with-rheumatoid-arthritis-passion-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-participation-in-the-adalimumab-humira-patient-support-program-on-functional-and-clinical-outcomes-among-patients-with-rheumatoid-arthritis-passion-study/