Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The flare-assessment in RA (FLARE) questionnaire was developed for the detection of disease activity flares in patients with rheumatoid arthritis (RA) based on the joint symptoms and systemic impact of RA disease. As of now, there is no clear FLARE score cut-off for identifying a flare in RA. We aimed to establish a threshold for RA flare using the FLARE questionnaire in patients with RA.
Methods: Patients with RA (age≥18 yrs; 2010 ACR criteria) participating in an ongoing prospective study completed the FLARE questionnaire on a monthly basis. The Health Assessment Questionnaire-II (HAQ-II), visual analogue scales (0-100 mm) for pain (VAS pain) and patient and provider global assessments of RA disease activity; assessment of tender (TJC28) and swollen joint counts of 28 joints (SJC28), and C-reactive protein (CRP) measurement were completed during the baseline visit. Generalized estimating equations were used to model the relationship between the FLARE questionnaire and patient report of flare incorporating random patient and visit effects to account for within patient variability.
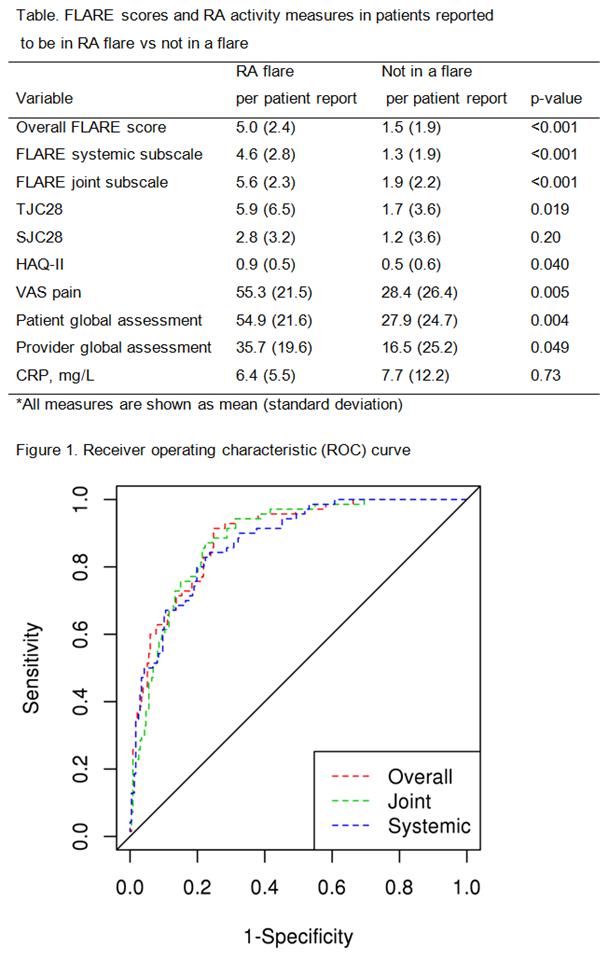
Results: The study included 66 patients with RA (mean age 59.7 years; 64% female). The mean (standard deviation, SD) for TJC28 joints was 2.3 (4.8), SJC28 was 1.1 (2.9), HAQ-II score 0.6 (0.5), VAS pain 33.3 (26.1), provider global assessment 17.2 (22.3), patient global assessment 33 (26.2) and CRP 7.7 (12) mg/L. A total of 307 monthly questionnaires were completed. As expected, the FLARE score overall, as well as systemic and joint subscale measures were significantly higher in months when patients reported flare vs no flare (Table). Several measures of RA activity including TJC28, HAQ-II, VAS pain, patient and provider global assessment were also significantly higher in patients reporting a RA flare vs those not reporting a flare (see the Table). Based on the ROC analysis, the optimal cutpoint (using the DeLong-Delong method) for the FLARE questionnaire was identified at 2.5. The sensitivity is 77.1% and the specificity is 78.9% using this cutpoint (Figure). The positive predictive value is 51.9% and the negative predictive value is 92.1%. The area under the curve was 89% for FLARE overall and 88% for both the joint and systemic subscales of the FLARE questionnaire.
Conclusion: We suggest a threshold of 2.5 for the FLARE questionnaire score to aid in the accurate identification of flare status in patients with RA. This threshold may be particularly useful in helping to rule out flare in RA patients where the diagnosis of flare is questionable, if their FLARE score is <2.5. Further studies are needed to validate this threshold in other populations of patients with RA. Future identification of an individualized threshold for RA flare using the FLARE questionnaire may aid in disease management.
To cite this abstract in AMA style:
Myasoedova E, Crowson CS, Davis JM III, Gabriel SE, Matteson EL. Identifying Flare in Rheumatoid Arthritis: What Is the Threshold? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/identifying-flare-in-rheumatoid-arthritis-what-is-the-threshold/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-flare-in-rheumatoid-arthritis-what-is-the-threshold/