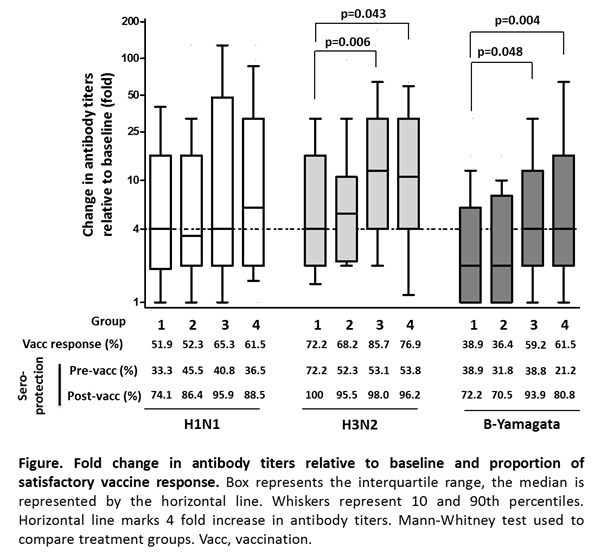Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Clinical Aspects III: Prevention of Comorbidity
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at a higher risk of infection due to the underlying immune dysfunction and immune suppression associated with treatment. Thus, vaccination against preventable diseases including influenza is recommended for all RA patients. Methotrexate (MTX), the mainstay of treatment in RA, has been associated with decreased response to influenza vaccination. This study was aimed to investigate whether a temporary discontinuation of MTX improves the vaccine efficacy to seasonal influenza.
Methods: In this single center, randomized, single-blind, open-label, prospective, parallel group intervention study, RA patients with stable MTX dose were randomly assigned to continue MTX (Group 1), to hold MTX for 4 weeks before vaccination (Group 2), to hold MTX for 2 weeks before and for 2 weeks after vaccination (Group 3) and to hold MTX for 4 weeks after vaccination (Group 4). We measured HI antibody titers against each antigen at baseline prior to vaccination with tri-valent seasonal influenza vaccination containing A/California/72009 (H1N1), A/Switzerland/9715293/2013 (H3N2) and B/Phuket/3073/2013 (B-Yamagata), and then again 4 weeks post-vaccination. Satisfactory vaccine response was defined as ¡Ã 4-fold increase in post-vaccination antibody titer relative to baseline. Protective antibody titer was defined as ¡Ã1:40.
Results: The per-protocol population comprised 219 patients (54, 44, 49 and 52 patients for Group 1, 2, 3 and 4, respectively). Prevaccination titer did not differ between the 4 groups. Four weeks after vaccination, Group 3 and Group 4 showed higher increase in antibody titers against H1N1 and H3N2 and B-Yamagata antigens than Group 1. The percentage of the satisfactory vaccine response against H3N2 and B-Yamagata were statistically higher in Group 3 and Group 4 than Group 1. By contrast, Group 2 were comparable to Group 1 in regard to increase in antibody titers and satisfactory vaccine response (Figure). The percentage of patients who showed protective titers for H1N1 and B-Yamagata was prominently increased in Group 3 and 4 as compared with group 1 (Figure). All groups showed high percentage of protective titers for H3N2. The influenza vaccine was well tolerated. RA flares were observed in 4 (7.4%), 6 (13.6%), 9 (18.4%) and 6 (11.5%) patients in Group 1, 2, 3 and 4, respectively, after vaccination.
Conclusion: Temporary discontinuation of MTX improves the immunogenicity of seasonal influenza vaccination in RA patients. Further studies are needed to determine the duration of MTX discontinuation. Trial registration: [www.clinicaltrials.gov, protocol number NCT02748785]
To cite this abstract in AMA style:
Park JK, Lee MAL, Lee KH, Lee EY, Song YW, Choi Y, Winthrop KL, Lee EB. Effect of Temporary Methotrexate Discontinuation on Efficacy of Seasonal Influenza Vaccination in Patients with Rheumatoid Arthritis: A Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/effect-of-temporary-methotrexate-discontinuation-on-efficacy-of-seasonal-influenza-vaccination-in-patients-with-rheumatoid-arthritis-a-randomized-clinical-trial/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-temporary-methotrexate-discontinuation-on-efficacy-of-seasonal-influenza-vaccination-in-patients-with-rheumatoid-arthritis-a-randomized-clinical-trial/