Session Information
Date: Monday, November 14, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment III: Novel and Current Therapies
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
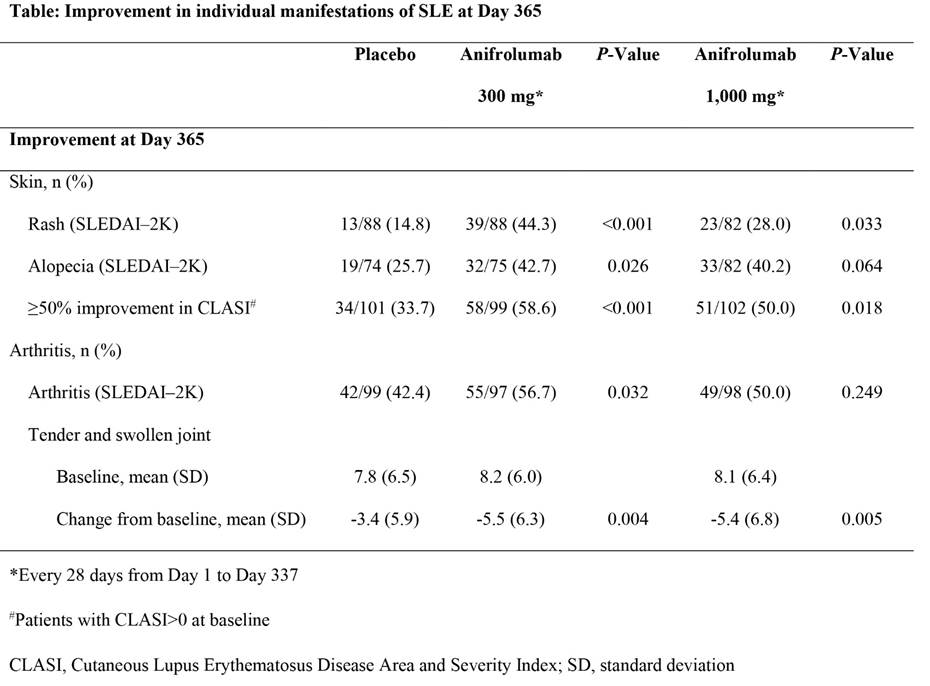
Background/Purpose: The Phase IIb MUSE study (NCT01438489) of intravenous anifrolumab in 305 patients with moderate to severely active SLE (300 or 1,000 mg vs. placebo, every 4 weeks for 48 weeks) demonstrated efficacy in multiple composite endpoints, with many favoring the 300 mg dose at Day 365.
Methods: This exploratory analysis compares the impact of anifrolumab on common individual manifestations of SLE including: rash, alopecia, and arthritis using the categorical descriptors of SLEDAI–2K and continuous outcome measures of cutaneous activity (rates of ≥50% improvement in the Cutaneous Lupus Erythematosus Disease Area and Severity Index [CLASI] activity score) and arthritis (change from baseline in swollen and tender joint counts).
Results: At Day 365, placebo response was minimal for SLEDAI–2K rash (Table), distinguishing a substantial treatment effect of anifrolumab, with 300 mg response rates markedly greater than 1,000 mg. More patients treated with anifrolumab compared with placebo also achieved ≥50% improvement in CLASI, but with minimal distinction between doses. The placebo group demonstrated response rates to alopecia and arthritis on SLEDAI–2K of 26% and 42%, respectively. A significantly greater response in alopecia and arthritis manifestations was achieved with 300 mg anifrolumab, with only modest drop-off in efficacy rates with 1,000 mg. Compared with placebo, decreased tender and swollen joint counts were greater in patients treated with anifrolumab, with no difference between doses. Anifrolumab effects on SLEDAI–2K improvement for each manifestation, were primarily seen in patients with a high type I interferon gene signature at baseline (IFN high), driven by lower placebo response rates in this subpopulation.
Conclusion: Anifrolumab demonstrated greater rates of improvement than placebo in rash, alopecia and joint manifestations, with greatest effects achieved by 300 mg anifrolumab in IFN-high patients. An apparent inverse dose response favoring the 300 mg (lower) dose, previously reported for several composite outcomes, characterized the SLEDAI–2K rash response which requires complete resolution of the rash, but was less pronounced in arthritis and alopecia categories or when using quantitative measures. These data contribute understanding of the impact of anifrolumab on individual lupus features, and suggests benefits of continuous variables, as demonstrated by the CLASI and joint counts, in outcome measures to inform interpretable trial designs.
To cite this abstract in AMA style:
Merrill J, Furie R, Werth VP, Khamashta M, Drappa J, Wang L, Illei G. The Effect of Anifrolumab on Cutaneous Manifestations and Arthritis in Moderate to Severe Systemic Lupus Erythematosus (SLE) Using Categorical SLEDAI–2K Responses and Continuous Measures of Activity As Outcome Measures [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-anifrolumab-on-cutaneous-manifestations-and-arthritis-in-moderate-to-severe-systemic-lupus-erythematosus-sle-using-categorical-sledai-2k-responses-and-continuous-measures-of-ac/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-anifrolumab-on-cutaneous-manifestations-and-arthritis-in-moderate-to-severe-systemic-lupus-erythematosus-sle-using-categorical-sledai-2k-responses-and-continuous-measures-of-ac/