Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Phenotypic, genetic, and treatment differences distinguish PR3- and MPO-ANCA+ ANCA-associated vasculitis (AAV) patients and suggest that these serotypes represent distinct conditions which might have different risk factors. Being overweight or obese is a risk factor in some autoimmune conditions (e.g., rheumatoid arthritis) but differences in body mass index (BMI) between the PR3- and MPO-ANCA+ AAV subtypes have not been investigated. Such differences could suggest novel genetic or environmental risk factors and have implications for treatment response, as in psoriatic arthritis, and the risk of cardiovascular disease which is a common cause of death in AAV. We evaluated whether BMI differences exist between patients with PR3-ANCA+ and MPO-ANCA+ AAV.
Methods: We analyzed AAV patients from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial. In univariate analyses, we compared the BMI of PR3- and MPO-ANCA+ AAV patients at enrollment; in a multivariate linear regression, we adjusted for age, gender, glucocorticoids prior to enrollment, relapsing disease at baseline, and baseline disease activity. We used an analysis of response profile to evaluate trends in BMI, adjusted for the above confounders as well as glucocorticoid exposure and flares during the trial.
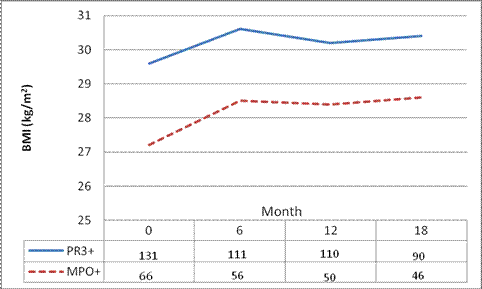
Results: The majority of patients in RAVE (N=197, Table 1) were PR3+ (131, 67%). PR3-ANCA+ patients had a higher baseline BMI (29.6±6.6 vs. 27.2 ± 5.3; P=0.01) and were more often obese (42% vs. 24%, P=0.04). When analyzing only those with a new diagnosis at enrollment (N=96), PR3-ANCA+ patients still had a significantly higher BMI (29.2 ± 6.2 vs. 26.8 ± 4.9, P=0.04). In multivariate regression analyses, these differences (PR3-ANCA+ BMI 2.1 kg/m2 (±1.1) higher than MPO-ANCA+ patients) remained (P=0.047). To address the potential impact of disease duration on BMI, we investigated the temporal trend in BMI between AAV subtypes over the course of the trial (18 months) and found a constant difference in BMI (Figure 1; P=0.3).
Conclusion: PR3-ANCA+ patients have a significantly higher BMI than MPO-ANCA+ patients, even after adjustment for important potential confounders. Several possibilities may explain findings. First, an elevated BMI may predispose to PR3-ANCA+ AAV. Second, the activity of hormones associated with metabolism (e.g., leptin) may differ in AAV subtypes. Third, BMI differences could be related to variations in shared genetic risk factors. Table 1: RAVE Trial Cohort
| Variable |
PR3-ANCA+ |
MPO-ANCA+ |
P-Value |
| Number (N, %) | 131 (66.5%) | 66 (33.5%) | |
| Age | 49.6 (±14.8) | 59 (±15) | <0.001 |
| Male (N, %) | 75 (57.3%) | 24 (36.4%) | 0.007 |
| Baseline BVAS-WG | 8.3 (±3.2) | 8.5 (±3.1) | 0.8 |
| New Diagnosis at Enrollment | 50 (38.2%) | 46 (70%) | <0.0001 |
| Disease Category | |||
| Granulomatosis with polyangiitis (N, %) | 127 (97%) | 20 (30%) | <0.0001 |
| Microscopic polyangiitis (N, %) | 4 (3%) | 44 (66.7%) | |
| Indeterminant (N, %) | 0 (0%) | 2 (3%) | |
| Weight and BMI | |||
| Baseline weight (kg) | 89.3 (±22.0) | 77.2 (±16.0) | <0.0001 |
| Baseline BMI (kg/m2) | 29.6 (±6.6) | 27.2 (± 5.3) | <0.0001 |
| Obese (N, %) | 55 (42%) | 16 (24%) | 0.04 |
| Glucocorticoid Dosing | |||
| GC prior to baseline (mg) | 1263 (±1492) | 1130 (±1408) | 0.6 |
| GC over the course of study (mg) | 4838.2 (±2001) | 4817.7 (±3433.1) | 0.96 |
Figure 1: Change in BMI During the RAVE Trial, Analysis of Response Profile
To cite this abstract in AMA style:
Wallace Z, Lu N, Miloslavsky E, Specks U, Hoffman GS, Kallenberg CGM, Langford CA, Merkel PA, Monach PA, Seo P, Spiera RF, St.Clair EW, Bruntetta P, Choi HK, Stone JH. Antineutrophil Cytoplasmic Antibody (ANCA) Type and Body Mass Index in ANCA-Associated Vasculitis (AAV) [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/antineutrophil-cytoplasmic-antibody-anca-type-and-body-mass-index-in-anca-associated-vasculitis-aav/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antineutrophil-cytoplasmic-antibody-anca-type-and-body-mass-index-in-anca-associated-vasculitis-aav/