Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Secukinumab (SEC) and infliximab (INF) are approved for the treatment of active PsA in adults with an inadequate response to conventional DMARDs. There are no head-to-head (H2H) randomized controlled trials (RCTs) between SEC and INF. In the absence of a H2H RCT, matching-adjusted indirect comparison (MAIC) can be used to generate comparative effectiveness data in the short and long term. MAIC adjusts for differences in baseline patient characteristics by using individual patient data (IPD) from one or more trials to match the treatment population of another trial. The aim of this MAIC was to assess the relative effectiveness of SEC 150 mg and 300 mg versus INF 5 mg/kg in patients with active PsA using data from the FUTURE 2 and IMPACT 2 RCTs.Methods: IPD from the SEC arms of FUTURE 2 (75 mg, n = 99; 150 mg, n = 100; 300 mg, n = 100) were weighted to match baseline characteristics of the INF arm of IMPACT 2 (n = 100); placebo arms were also matched (FUTURE 2, n = 98; IMPACT 2, n = 100). Logistic regression was used to determine weights for age, body weight, sex, race, methotrexate use, presence of psoriasis on ≥ 3% of body surface area, mean PASI score, Health Assessment Questionnaire Disability Index (HAQ-DI) score, dactylitis, enthesitis and previous use of anti-TNFs. Recalculated outcomes from FUTURE 2 (150 mg effective sample size [ESS]: 31; 300 mg ESS: 34; placebo ESS: 15) were compared with published aggregate outcomes from IMPACT 2 at weeks 14/16, 24 and 54/52 (the closest data points available for comparison). Placebo-adjustment was valid only until week 14/16 because patients could switch to active treatment as early as week 16 in both trials. ACR outcomes are presented as response rates (%) and pairwise comparisons using odds ratios (ORs).
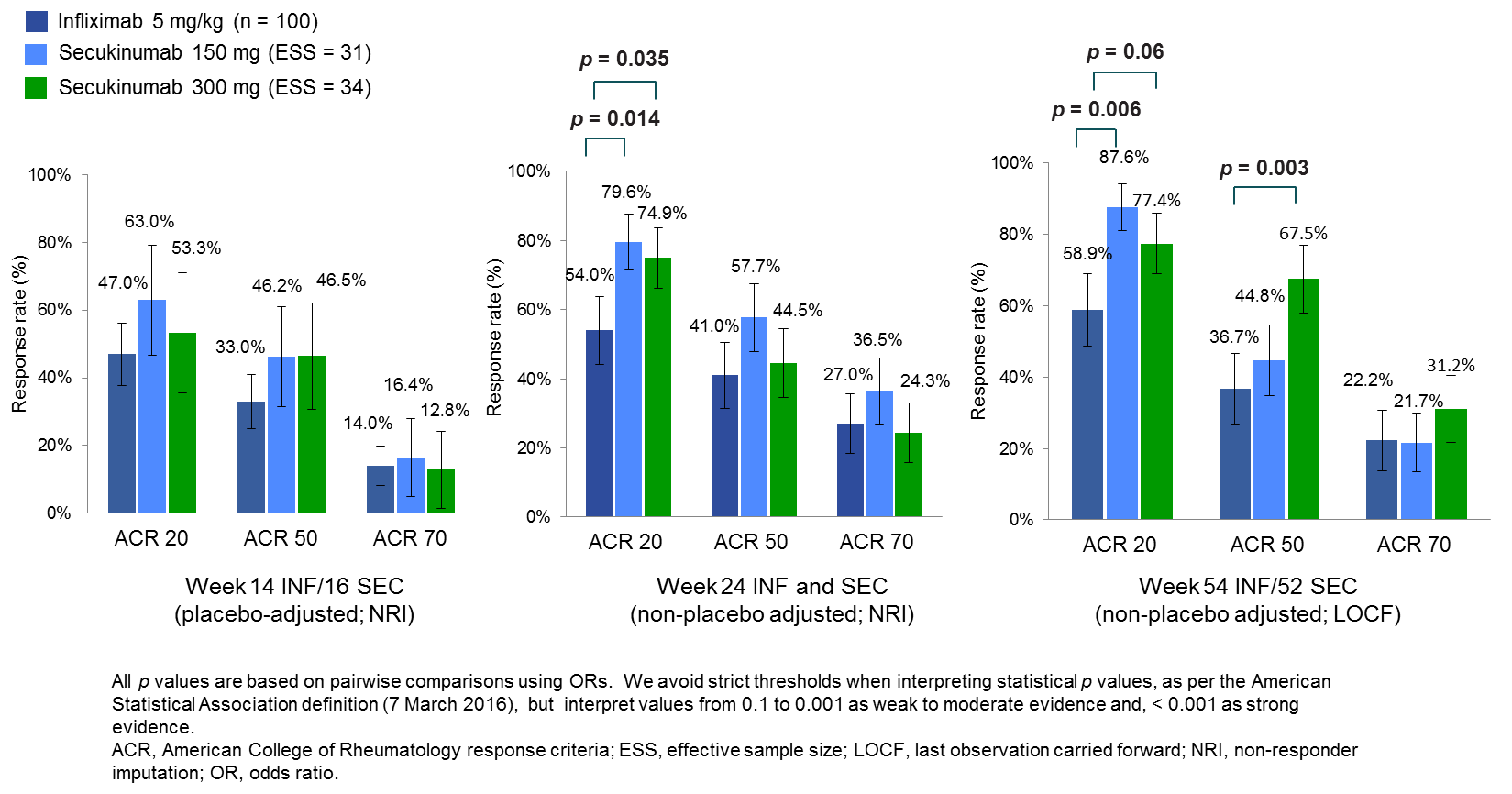
Results: The ACR 20 response at week 14/16 with placebo was 8% in FUTURE and 11% in IMPACT 2, indicating a good match. After placebo-adjustment, at week 14/16 there was no difference in ACR 20 response rate between SEC and INF. At week 24, there was evidence of higher ACR 20 response rates for SEC 150 mg and 300 mg than for INF (OR [95% CI]: 3.33 [1.28–8.69]; p = 0.014 and OR: 2.55 [1.07–6.08]; p = 0.035, respectively). At week 54/52, there was evidence of a higher ACR 20 response rate for SEC 150 mg (OR [95% CI]: 4.92 [1.56–15.48]; p = 0.006) and a higher ACR 50 response rate for SEC 300 mg (OR [95% CI]: 3.59 [1.56–8.29]; p = 0.003) than for INF.
Conclusion: This MAIC showed higher ACR 20 response rates for SEC 150 mg and 300 mg than for INF at week 24, and higher ACR 20 (150 mg) and 50 (300 mg) response rates than for INF (non-placebo-adjusted) at week 54/52. There was no evidence of differences in the short-term placebo-adjusted phase. Key limitations of this MAIC are that placebo-adjustments were valid only until week 14/16, week 24 was the only common time point for which outcomes were reported for both RCTs, and the ESS for SEC was low. H2H RCTs remain the gold standard for comparing treatments.
MAIC ACR response rates at weeks 14/16, 24 and 54/52
To cite this abstract in AMA style:
Strand V, Mease PJ, McInnes IB, Nash P, Thom H, Hunger M, Gandhi K, Mpofu S, Jugl S. Secukinumab for the Treatment of Psoriatic Arthritis: Comparative Effectiveness Versus Infliximab Using a Matching-Adjusted Indirect Comparison [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/secukinumab-for-the-treatment-of-psoriatic-arthritis-comparative-effectiveness-versus-infliximab-using-a-matching-adjusted-indirect-comparison/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-for-the-treatment-of-psoriatic-arthritis-comparative-effectiveness-versus-infliximab-using-a-matching-adjusted-indirect-comparison/