Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pharmacokinetic (PK) equivalence was demonstrated, and similar safety profiles of CT-P10 to EU-sourced innovator rituximab (EU-RTX) were shown in the phase 1 studies up to 2 years in RA patients including switching to CT-P10 from EU-RTX.1,2 The purpose of this study was to demonstrate efficacy equivalence and compare safety profiles of CT-P10 to reference products (combined EU and US–sourced RTX) in RA patients up to 24 weeks.
Methods: In this randomized, controlled phase 3 study, RA patients were randomized to receive CT-P10 or reference products (NCT02149121). The primary efficacy endpoint, change of DAS28–CRP from baseline to week 24, was evaluated and analyzed by using an analysis of covariance (ANCOVA). Therapeutic equivalence is to be concluded if the 95% confidence interval (CI) for the treatment difference in the change of DAS28-CRP from baseline to Week 24 is entirely within the pre-specified equivalence margin of +/-0.60. Additional efficacy, pharmacodynamics (PD) and safety were also evaluated.
Results: A total of 372 RA patients (161 patients and 211 patients in CT-P10 and reference products groups, respectively) were enrolled. Overall efficacy, PD and safety profiles were similar between CT-P10 and reference products groups. The adjusted mean change of DAS28-CRP/ESR from baseline to week 24 was similar between the groups. The 95% CI for the estimate of treatment difference in DAS28-CRP/ESR was entirely within the equivalence margin which indicated therapeutic equivalence between the treatment groups (Table 1). Additional efficacy including ACR and EULAR responses was also shown to be comparable between 2 groups (Table 2). Rapid and complete depletion of B-cell counts were observed immediately after the first infusion, and B-cell kinetics over 24 weeks were similar between the groups. Adverse events (AEs) related to study drug were reported with a similar proportion in each treatment group; 49 (30.4%) and 59 (28.0%) patients in CT-P10 and reference products groups, respectively. Infection related to study drug was reported in 13 (8.1%) and 22 (10.4%) patients in CT-P10 and reference products groups, respectively. No malignancy, progressive multifocal leukoencephalopathy, and serious infusion-related reaction were reported. 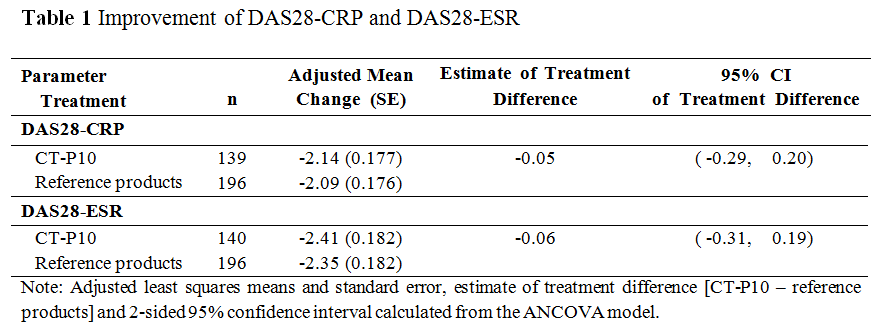

Conclusion: CT-P10 showed highly similar efficacy, PD and safety profiles to reference products up to 24 weeks. Reference
1. Yoo DH, et al. Arthritis Rheum 2013;65(Suppl 10): S736
2. Yoo DH, et al. Arthritis Rheum 2015;67(Suppl 10): 2449-2452
To cite this abstract in AMA style:
Yoo DH, Bozic Majstorovic L, Berrocal Kasay A, Chalouhi El-Khouri E, Irazoque-Palazuelos F, Cons Molina F, Miranda P, Shesternya P, Medina-Rodriguez FG, Wiland P, Jeka S, Garmish O, Hrycaj P, Rekalov D, Fomina N, Zisman D, Park YB, Kang YM, Suh CH, Shim SC, Lee SJ, Lee SY, Park W. Efficacy and Safety of CT-P10, Rituximab Biosimilar Candidate, and Innovator Rituximab in Patients with Rheumatoid Arthritis: Results from Phase 3 Randomized Controlled Trial over 24 Weeks [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-ct-p10-rituximab-biosimilar-candidate-and-innovator-rituximab-in-patients-with-rheumatoid-arthritis-results-from-phase-3-randomized-controlled-trial-over-24-weeks/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-ct-p10-rituximab-biosimilar-candidate-and-innovator-rituximab-in-patients-with-rheumatoid-arthritis-results-from-phase-3-randomized-controlled-trial-over-24-weeks/
