Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Skin fibrosis is the hallmark of SSc and may lead to significantly reduced quality of life in ways that may not be directly proportional to the area and severity of skin involvement such as that captured by the modified Rodnan skin score (mRSS). We developed a patient reported outcome instrument (PRO) to assess specifically the skin-related quality of life in patients with SSc for use in routine practice and clinical trials.
Methods: We conducted 3 focus groups (N=12) with SSc participants on how their skin condition affected their lives. Analysis of transcripts resulted in themes, which were conceptually encapsulated into four constructs: physical symptoms, physical limitations, emotional effects and social effects. 56 items for the Scleroderma Skin PRO (SSPRO) were created or adapted from existing PROs to assess each of these constructs. Input from an expert panel as well as cognitive interviews with 10 SSc participants led to removal of items with 22 remaining. The 22-item SSPRO was administered to 140 participants who also completed other PROs and rated their global skin severity. An mRSS and a global disease severity rating were also completed by a physician. Psychometric analysis included test-retest reliability, internal consistency, construct validity, exploratory and confirmatory factor analyses.
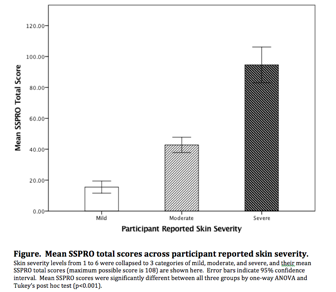
Results: Participants (N=140) primarily had lcSSc (67.1%), were female (82.1%) and Caucasian (92.9%) with mean age of 53.4 years and disease duration of 6.4 years. Mean mRSS was 9.3 (SD 10.8). Self-reported skin severity was mild (45%), moderate (48.5%), and severe (5.7%). Factor analysis supported 4 underlying factors in the SSPRO corresponding to the 4 hypothesized constructs. Removal of 4/22 items that contributed most to cross loading resulted in acceptable fit statistics in a confirmatory analysis. Test-retest reliability was moderate to high (ICC = 0.61–0.83) and internal consistency (CronbachÕs alpha = 0.89-0.96) was high. SSPRO correlated strongly with other patient reported measures suggesting construct validity, and less well with physician assessed outcomes (Table). SSPRO scores were also able to discriminate between participant-reported skin severity levels (Figure), as well as between limited and diffuse disease providing further evidence of construct validity.
Conclusion: SSPRO has been developed with extensive patient input and demonstrates reliability as well as face and construct validity. It is complementary to existing measures of SSc skin involvement with emphasis on the patientÕs experience. Further research is needed to assess its sensitivity to change. 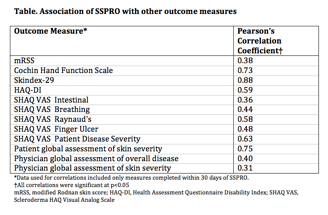
To cite this abstract in AMA style:
Man A, Correa JK, Ziemek J, Felson DT, Lafyatis R. Development of a Skin-Specific Scleroderma Patient Reported Outcome Instrument [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/development-of-a-skin-specific-scleroderma-patient-reported-outcome-instrument/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-skin-specific-scleroderma-patient-reported-outcome-instrument/