Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: We recently reported that real-world persistence in Veteran’s Affairs (VA) patients was lower in Rheumatoid arthritis (RA) patients receiving triple therapy [methotrexate (MTX), sulfasalazine (SUL) and hydroxychloroquine (HCQ)] at 17.5% in comparison to patients receiving MTX-Tumor necrosis factor inhibitor (TNFi) combination therapy at 45.2%. (p<0.001). This study reports and compares the specific reasons for discontinuation of these two combination regimens.
Methods: In this historical cohort study, US Veterans with RA enrolled in VA between January 1, 2006 to December 31, 2012 were identified using clinical and administrative data. Patients receiving MTX monotherapy to which either a TNFi (MTX-TNFi) or the simultaneous addition of HCQ/SUL (triple Rx) were followed over 12 months after initiating the combination. Discontinuation of combination therapy was defined as discontinuing any of the combination medications with a >90day gap and/or starting new drug treatments for RA. Patients discontinuing triple Rx or MTX-TNFi were matched to each other on age (±5 years), sex and site of care. The specific drugs discontinued and the reasons for discontinuing the combination therapy were determined by medical record review with the reason for cessation classified as one of the following: lack of efficacy, adverse drug event, or other (step-down therapy, preoperative discontinuation, noncompliance, lost to follow-up).
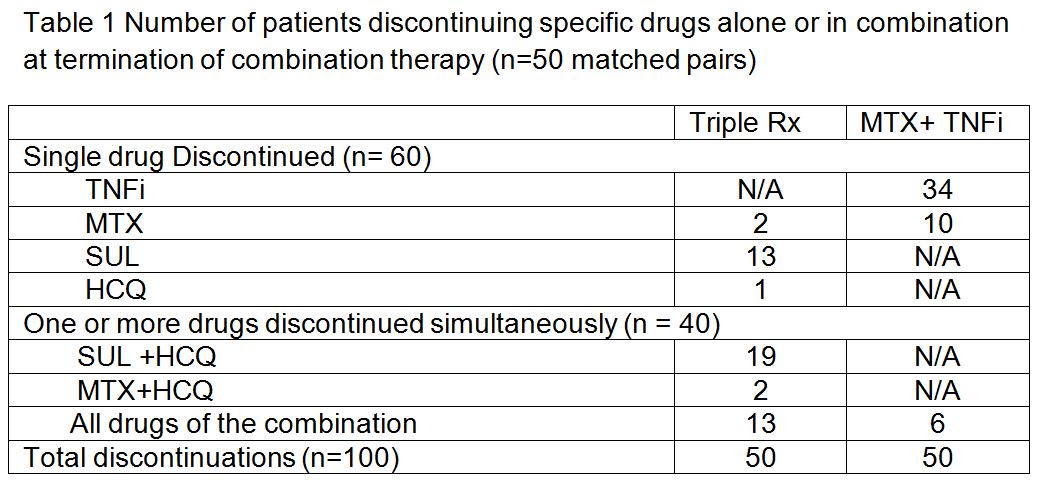
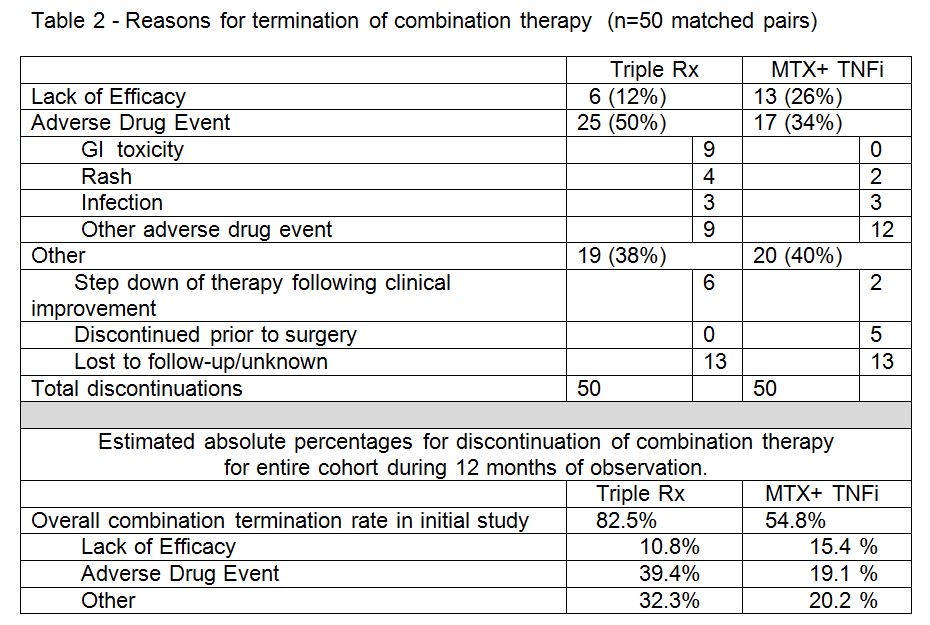
Results: There were 50 matched pairs between the two combination therapy groups evaluated. The drugs discontinued (table 1) and reasons for discontinuation (table 2) are shown below. The absolute rates for discontinuation by cause were estimated by extrapolating data over the entire cohort for the two combination treatment groups. Comparing discontinuation of triple therapy discontinuation of MTX-TNFi, the triple Rx group was associated with more discontinuations due to adverse drug events by both absolute and relative comparisons. Discontinuation due to lack of efficacy and other reasons for discontinuation were similar for the two groups. Methotrexate had the lowest discontinuation frequency in either group, with sulfasalazine being the most common drug discontinued in the triple Rx group.
Conclusion: The differences in persistence between the MTX-TNFi and Triple Rx groups in this real world observation cohort study appear to be primarily related to adverse drug events. The most common drug associated with adverse drug events was sulfasalazine, which was also the drug most frequently discontinued.
To cite this abstract in AMA style:
Erhardt D, Sauer BC, Teng CC, Mikuls TR, Curtis JR, Cannon GW. The Reasons for Discontinuation of Combination Therapy with Methotrexate and Tumor Necrosis Factor Inhibitors Versus Triple Therapy Differ Significantly Because of Higher Adverse Events with Triple Therapy [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-reasons-for-discontinuation-of-combination-therapy-with-methotrexate-and-tumor-necrosis-factor-inhibitors-versus-triple-therapy-differ-significantly-because-of-higher-adverse-events-with-trip/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-reasons-for-discontinuation-of-combination-therapy-with-methotrexate-and-tumor-necrosis-factor-inhibitors-versus-triple-therapy-differ-significantly-because-of-higher-adverse-events-with-trip/