Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose : SB5 has been developed as a biosimilar of the reference adalimumab (ADL). The 52-week efficacy and safety results were reported previously. 1 Here we report the efficacy results by anti-drug antibody (ADA) during the transition period.
Methods : Patients with moderate to severe rheumatoid arthritis (RA) despite methotrexate treatment were randomly assigned to receive 40 mg of either SB5 or ADL administered subcutaneously every other week. After 24 weeks of treatment, patients in the ADL group were re-randomized (1:1) to either be transitioned to SB5 (ADL/SB5) or continue on ADL (ADL/ADL) up to Week 50. Patients receiving SB5 continued to receive SB5 (SB5/SB5) but they followed the randomization procedure for blinding purposes. Efficacy, safety, and immunogenicity were assessed up to Week 52. Patients with detectable ADA were those who newly developed ADA or developed ADA with increased titer after transition.
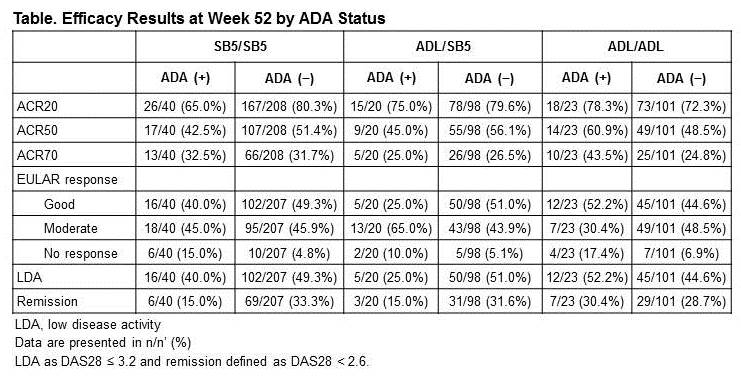
Results : The proportion of patients with detectable ADA after transition was 15.7% vs. 16.8% vs. 18.3% for SB5/SB5, ADL/SB5, and ADL/ADL, respectively. In all treatment groups, the mean DAS28 tended to improve in patients without ADA while it tended to worsen in patients with detectable ADA between Week 24 and Week 52 (Figure). The mean change in DAS28 from Week 24 was comparable across treatment groups within each ADA subgroup. In other efficacy parameters such as EULAR response rates, proportion of patients with low disease activity and remission based on DAS28, there was a trend towards decreased efficacy in patients with detectable ADA compared to those without ADA (Table).
Conclusion : Patients with detectable ADA after transition were more likely to have reduced efficacy compared to those without ADA. Efficacy was comparable across treatment groups within patients with detectable ADA and within patients without ADA.
Reference 1. Weitnblatt ME et al. Ann Rheum Dis 2016;75(Suppl2): 487
To cite this abstract in AMA style:
Genovese MC, Weinblatt M, Keystone EC, Baranauskaite A, Cheong SY, Ghil J. Efficacy after Transition to SB5 from Reference Adalimumab (Humira®) Vs. Continuation of SB5 or Reference Adalimumab By Antibodies Developed after Transition from a SB5 Phase III Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-after-transition-to-sb5-from-reference-adalimumab-humira-vs-continuation-of-sb5-or-reference-adalimumab-by-antibodies-developed-after-transition-from-a-sb5-phase-iii-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-after-transition-to-sb5-from-reference-adalimumab-humira-vs-continuation-of-sb5-or-reference-adalimumab-by-antibodies-developed-after-transition-from-a-sb5-phase-iii-study/