Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
SB5 has been developed as a biosimilar of the reference adalimumab (ADL). The 24-week results of the phase III study have been reported.1 Efficacy, safety, and immunogenicity results in the transition period (Week 24 to Week 52) will be presented.Methods:
Patients with RA were randomized in a 1:1 ratio to receive either 40 mg of SB5 or ADL every other week with background methotrexate. After 24 weeks of treatment, patients in the ADL group were re-randomized (1:1) to either be transitioned to SB5 (ADL/SB5) or continue on ADL (ADL/ADL) up to Week 50. Patients receiving SB5 continued to receive SB5 (SB5/SB5) but they followed the randomization procedure for blinding purposes. Efficacy, safety, and immunogenicity were assessed up to Week 52.Results:
After 24-weeks of treatment, 254 patients from SB5 continued to receive SB5 (SB5/SB5), 125 patients from ADL were transitioned to SB5 (ADL/SB5), and 129 patients from ADL continued to receive ADL (ADL/ADL). Baseline characteristics at baseline and disease activities at re-randomization were comparable across treatment groups. The ACR responses were sustained and comparable across treatment groups during the transition period in the full analysis set (Figure). At Week 52 the ACR20 response rates were 76.9% vs. 81.1% vs. 71.2% in SB5/SB5, ADL/SB5, and ADL/ADL in the per-protocol set. Other efficacy endpoints including radiographic change were also comparable across the treatment groups. The mean change from baseline in modified Total Sharp Score was 0.17 in SB5/SB5 vs. 0.25 in ADL/SB5 vs. 0.50 in ADL/ADL. The safety profile during the transition period was comparable across treatment groups. The proportion of patients with any treatment-emergent adverse events after transition was 32.3% in SB5/SB5, 37.6% in ADL/SB5, and 33.1% in ADL/ADL and two patients in ADL/ADL group only newly reported injection site reactions. The incidence of anti-drug antibody after transition was 15.7% vs. 16.8% vs. 18.3% in SB5/SB5, ADL/SB5, and ADL/ADL, respectively.Conclusion:
Efficacy, safety, and immunogenicity profiles were comparable between SB5/SB5, ADL/SB5, and ADL/ADL during the transition period (Week 24 to Week 52). There were no treatment emergent issues or clinically relevant immunogenicity precipitated by switching.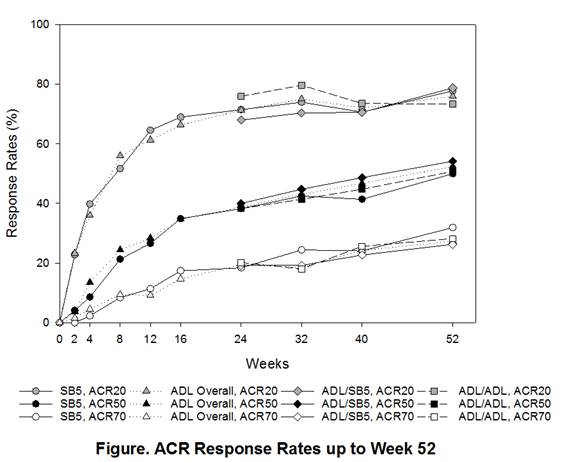
Reference 1. Weinblatt ME et al. Arthritis Rheumatol. 2015; 67 (suppl 10), 8L
Disclosure: M. Weinblatt, Amgen, BMS, Crescendo Bioscience, UCB, 2,Amgen, AbbVie, BMS, Eli-Lilly, Gilead, Merck, Pfizer Inc, Novartis, Roche, Samsung Bioepis, UCB, 5; A. Baranauskaite, Abbvie, Samsung Bioepis, 5; J. Niebrzydowski, Samsung Bioepis, 2; E. Dokoupilova, Samsung Bioepis, 2; A. Zielinska, Samsung Bioepis, 2; K. Sitek-Ziolkowska, Samsung Bioepis, 2; J. Jaworski, Samsung Bioepis, 2; A. Racewicz, Samsung Bioepis, 2; M. Pileckyte, Samsung Bioepis, 2; K. Jedrychowicz-Rosiak, Samsung Bioepis, 2; V. Zhdan, Samsung Bioepis, 2; S. Y. Cheong, Samsung Bioepis, 3; J. Ghil, Samsung Bioepis, 3.
To cite this abstract in AMA style:
Weinblatt M, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Sitek-Ziolkowska K, Jaworski J, Racewicz A, Pileckyte M, Jedrychowicz-Rosiak K, Zhdan V, Cheong SY, Ghil J. Sustained Efficacy and Comparable Safety and Immunogenicity after Transition to SB5 (an Adalimumab Biosimilar) Vs. Continuation of SB5 or Reference Adalimumab (Humira®) in Patients with Rheumatoid Arthritis: Results of Phase III Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/sustained-efficacy-and-comparable-safety-and-immunogenicity-after-transition-to-sb5-an-adalimumab-biosimilar-vs-continuation-of-sb5-or-reference-adalimumab-humira-in-patients-with-rheumatoi/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-efficacy-and-comparable-safety-and-immunogenicity-after-transition-to-sb5-an-adalimumab-biosimilar-vs-continuation-of-sb5-or-reference-adalimumab-humira-in-patients-with-rheumatoi/
