Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Giant cell arteritis (GCA) is characterized by a destructive, granulomatous inflammation in the walls of medium and large-sized arteries. Glucocorticoid (GC) treatment controls symptoms and reduces the risk of vascular complications such as vessel occlusion with blindness or rupture with fatal bleeding. Unfortunately, the duration of treatment and frequent relapses lead to high cumulative GC doses with substantial toxicity and morbidity. Tocilizumab (TCZ) is a humanized IgG1k monoclonal antibody directed against the human interleukin-6 receptor, it has been shown to rapidly induce remission in case series. This RCT was designed to evaluate the efficacy and safety of TCZ in the induction and maintenance of disease remission in patients with newly diagnosed or recurrent GCA.
Methods: Single center, randomized, placebo-controlled trial. Participants satisfying the 1990 ACR criteria were randomly assigned between 2011 and 2014 in a 2:1 ratio to receive TCZ (8 mg/kg IV of body weight) + GC (PO) or placebo (IV) + GC (PO). Infusions were given every 4 weeks for 1 year. GC was started at 1mg/kg/d and tapered by 0.1mg/kg/d weekly until week 8, then by 0.05mg/kg/d weekly until week 12 (0.1mg/kg/d). Thereafter, the dosage was decreased by 1mg/d monthly to 0mg.
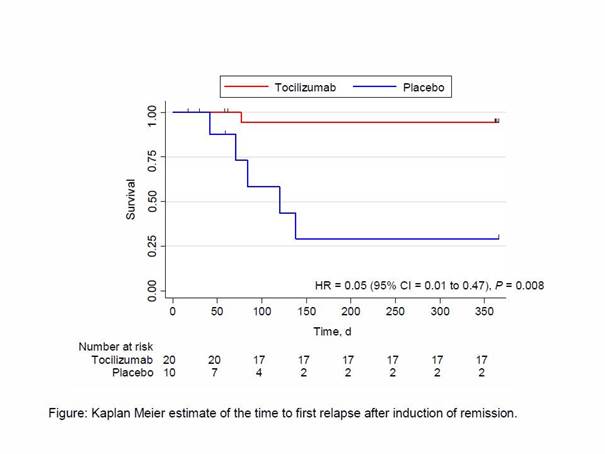
The primary outcome was the number of patients with complete remission at week 12 (GC dose of 0.1mg/kg/d), reported as crude risk difference and corresponding 95% confidence intervals (CI). P-values were derived by Fisher’s exact test. The secondary outcome was the number of patients who remained relapse-free at week 52 while the GC dosage was being tapered, plotted as a Kaplan-Meier curve for relapse-free survival for each group. Groups were compared with a Cox proportional hazard model.
Results: Thirty patients, 70% of whom were female were randomized (20 assigned to TCZ + GC, and 10 to Placebo +GC). Sixteen (80%) patients in the TCZ group and 7 (70%) in the placebo group were newly diagnosed. After 12 weeks, 17 patients in the TCZ group and 4 in the placebo group were in complete remission, (85% vs. 40%, respectively; difference, 45 percentage points [95% CI = 11 to 79 percentage points]; P = 0.030). At the end of the trial, 17 in the TCZ group and 2 patients in the placebo group had experienced no relapse (85% vs. 20%, respectively; difference, 65 percentage points), with a corresponding hazard ratio = 0.05 (95% CI 0.01 to 0.47), P = 0.008 (Figure).
Conclusion: This trial demonstrates the efficacy of TCZ for induction and maintenance therapy in patients with newly diagnosed or relapsed GCA in the context of rapidly tapered glucocorticoids.
To cite this abstract in AMA style:
Adler S, Reichenbach S, Kuchen S, Wermelinger F, Dan D, Villiger PM, Seitz M. Tocilizumab for the Treatment of Giant Cell Arteritis – a Randomized Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/tocilizumab-for-the-treatment-of-giant-cell-arteritis-a-randomized-placebo-controlled-trial/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-for-the-treatment-of-giant-cell-arteritis-a-randomized-placebo-controlled-trial/