Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis-Small Molecules, Biologics and Gene Therapy VI: Strategies
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: In early rheumatoid arthritis (eRA), a clinically significant
proportion of patients may respond to first-line treatment with methotrexate
(MTX). A priori identification of patients with high or low likelihood of
response to MTX would enhance therapy strategies. Previously, we have presented
data on the multi-biomarker disease activity (MBDA) score, based on twelve
biomarkers, from the Swedish Farmacotherapy (SWEFOT) trial. The objective of
this study was to investigate these biomarkers at baseline (BL) separately and
in simple combinations as predictors of response to MTX monotherapy.
Methods: Analyses
were done on a subset of 298 patients with eRA from the SWEFOT population (104
responders [DAS28≤3.2] and 194 non-responders [DAS28>3.2] to MTX) who
had complete data on the 12 biomarkers from the MBDA score at BL and DAS28 at
Month 3. The categories of these biomarkers (low, moderate and high) were
defined using tertiles with the exception of CRP, where the following cutoffs (mg/L)
were applied: ≤10 for low, >10-30 for moderate and >30 for high.
For the comparison of proportions of responders and non-responders between
patients with different categories of the biomarkers, the χ2
test was used. Each individual biomarker was analyzed as a predictor (without
correcting for multiple comparisons), followed by a study of combinations of
the most strongly predictive biomarkers.
Results: In
MTX-responders versus non-responders, out of the 12 biomarkers at BL, the
medians of CRP and IL-6 were significantly lower (15 vs 20, p=0.038, and 49 vs.
67, p=0.049), and TNF-RI and VCAM-1 were significantly higher (1.9 vs 1.7,
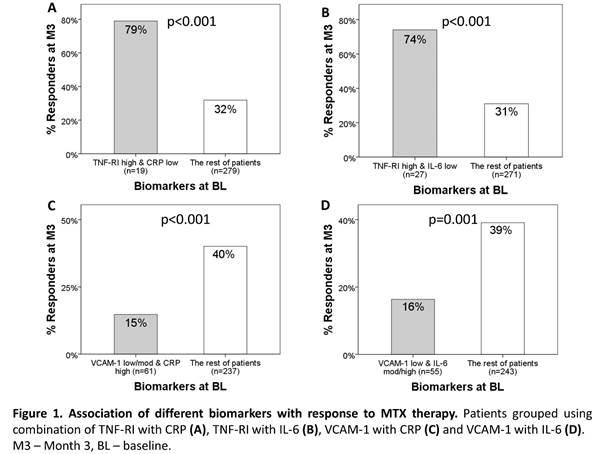
p=0.005, and 0.70 vs. 0.64, p=0.006), respectively. Of patients with both low
CRP AND high TNF-RI at BL (n=19), or low IL-6 AND high TNF-RI (n=27) higher
proportions were responders compared with the rest (79% vs 32%, p<0.001, and
74% vs 31%, p<0.001, Figures 1A and B respectively). In contrast, patients
with low/moderate VCAM-1 AND high CRP (n=61) or low VCAM-1 AND moderate/high IL-6
(n=55) were more likely not to respond to MTX than the others (85% vs 60%,
p<0.001; Figures 1C and D). All 19 patients with low VCAM-1 AND high CRP AND
moderate/high IL-6 were MTX-non-responders.
Conclusion: We
identified individual biomarkers and 2-biomarker combinations that were
associated, positively or negatively, with the clinical response to MTX
monotherapy. If reproduced in other study populations, these results suggest
that biomarkers and their combinations might be helpful in decision-making on
the initial therapy of patients with early RA.
To cite this abstract in AMA style:
Hambardzumyan K, Bolce RJ, Saevarsdottir S, Forslind K, Karlsson JA, van Vollenhoven RF. Predictive Biomarkers for Response or Non-Response to MTX Monotherapy in Early RA [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/predictive-biomarkers-for-response-or-non-response-to-mtx-monotherapy-in-early-ra/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictive-biomarkers-for-response-or-non-response-to-mtx-monotherapy-in-early-ra/