Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Scalp and nail psoriasis are
difficult-to-manage manifestations of psoriasis and psoriatic arthritis (PsA). Nail involvement
occurs in ~80% of pts with PsA and can negatively
affect daily/work activities, thereby impacting pt quality of life. ESTEEM 1
and 2 are phase 3, randomized, controlled trials evaluating apremilast (APR) efficacy/safety
in pts with moderate to severe plaque psoriasis. Of 1,255 pts randomized in the
ESTEEM trials, 65.7% had nail and 66.3% had moderate to severe scalp
involvement at baseline (BL).
Methods: Pts were randomized 2:1 to APR 30 mg BID (APR30) or
placebo (PBO). At Wk 16, PBO pts switched to APR30. All pts were treated with
APR30 through Wk 32, followed by a randomized treatment withdrawal phase up to
52 wks in pts randomized to APR30 at BL and identified as responders at Wk 32. The
primary endpoint was the proportion of pts achieving PASI-75 at Wk 16. Nail and
scalp involvement were assessed through Wk 52 in pts with Nail Psoriasis
Severity Index (NAPSI) ≥1 in the target nail and Scalp Physician Global
Assessment (ScPGA) ≥3 at BL.
Results: Improvements in
mean PASI score occurred as early as Wk 2 in ESTEEM 1 and 2 with APR30 (-22.3%
and -24.3%, respectively) vs PBO (-6.5% and -7.5%). At Wk 16,
significantly more pts achieved PASI-75 in ESTEEM 1 and 2 with APR30 (33.1% and
28.8%, respectively) vs PBO (5.3% and 5.8%; both P<0.0001). Improvements
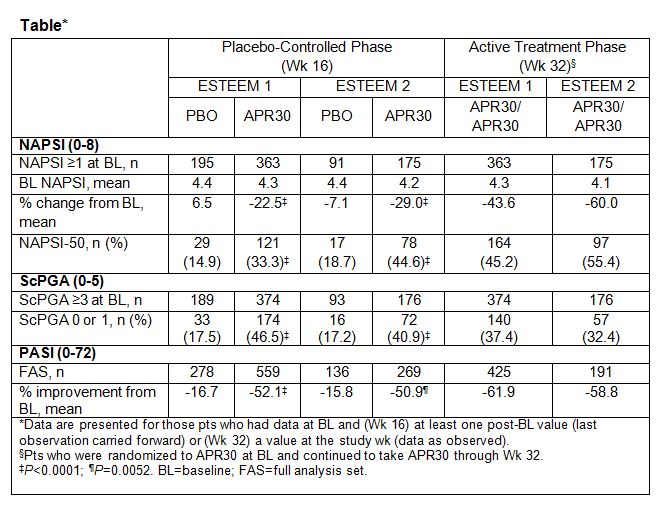
in mean percent change in NAPSI score and NAPSI-50 achievement rates were also
significantly greater with APR30 vs PBO at Wk 16 (Table). Similarly, a higher
proportion of pts achieved an ScPGA of 0 (clear) or 1 (minimal) at Wk 16 with
APR30 (P<0.0001, both studies; Table). At Wk 32, mean percent change
in NAPSI as well as NAPSI-50 and ScPGA 0 or 1 achievement were sustained in pts
randomized to and continuing treatment with APR30 (Table). Among pts randomized
to APR30 at BL who were randomized to continue APR30 (with no addition of other
treatments) through Wk 52, mean improvement in PASI score at Wk 52 was –80.5%
in ESTEEM 1 and –74.4%
in ESTEEM 2. Improvements in NAPSI outcomes were maintained in these pts over
52 wks: mean percent change in NAPSI was –60.2%
in ESTEEM 1 and –59.7%
in ESTEEM 2; achievement of NAPSI-50 was 70.7% and 68.6%, respectively. In this
pt group, ScPGA 0 or 1 was reached by 83.3% of pts in ESTEEM 1 and 62.5% of pts
in ESTEEM 2. AEs occurring in ≥5% of pts treated with APR30 were diarrhea,
nausea, URTI, nasopharyngitis, tension headache, and headache up to 16 wks. Most
AEs were mild or moderate in severity, with no increase in incidence or
severity with up to ≥52 wks of treatment.
Conclusion: In the ESTEEM
plaque psoriasis studies, APR30 was effective in improving skin involvement and
the difficult-to-manage manifestations of nail and scalp psoriasis, with
maintenance of these improvements over time. APR30 was generally well
tolerated, with the most common tolerability issues occurring early and
resolving with continued treatment.
To cite this abstract in AMA style:
Papp K, Crowley J, Paul C, Gooderham M, Reich K, Hu C, Day RM, Griffiths CEM. Apremilast, an Oral Phosphodiesterase 4 Inhibitor: Improvements in Nail and Scalp Psoriasis and Psoriasis Area and Severity Index in Patients with Moderate to Severe Plaque Psoriasis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/apremilast-an-oral-phosphodiesterase-4-inhibitor-improvements-in-nail-and-scalp-psoriasis-and-psoriasis-area-and-severity-index-in-patients-with-moderate-to-severe-plaque-psoriasis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-an-oral-phosphodiesterase-4-inhibitor-improvements-in-nail-and-scalp-psoriasis-and-psoriasis-area-and-severity-index-in-patients-with-moderate-to-severe-plaque-psoriasis/