Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Previous reports of RAPID-PsA (NCT01087788) have
demonstrated the efficacy of certolizumab pegol (CZP) in patients (pts) with
psoriatic arthritis (PsA) over 96 weeks (wks),1 and have shown that
improvements in ACR responses and skin manifestations were similar regardless
of prior anti-TNF exposure.2 Here we report the efficacy of CZP for
the treatment of joint and extra-articular manifestations (EAMs) of PsA in pts
with and without prior anti-TNF exposure over 96 wks of the RAPID-PsA trial.
Methods:
RAPID-PsA was double-blind and placebo-controlled to
Wk24, dose-blind to Wk48 and open-label to Wk216. Pts had active PsA, had
failed ≥1 DMARD, and ≤40% pts could have received 1 prior anti-TNF.
409 pts were randomized at baseline (BL) to either placebo or CZP (200 mg
Q2W/400 mg Q4W, following 400 mg loading dose at Wks 0, 2, 4). The primary
clinical endpoint was Wk12 ACR20 response.3 Joint disease activity
was assessed in all pts using the tender joint count (TJC), swollen joint count
(SJC) and DAS28(CRP). EAMs were assessed in pts with BL involvement and
included nail psoriasis (modified nail psoriasis severity index [mNAPSI], BL
involvement = BL mNAPSI >0; for some pts the nail analyzed was different at
one or more visit to Wk96), enthesitis (Leeds enthesitis index [LEI], BL
involvement = BL LEI >0) and dactylitis (Leeds dactylitis index [LDI], BL
involvement = at least 1 digit affected and with a difference in circumference ≥10%
compared to the contralateral digit). Observed data are presented for all pts
originally randomized to CZP with and without prior anti-TNF exposure.
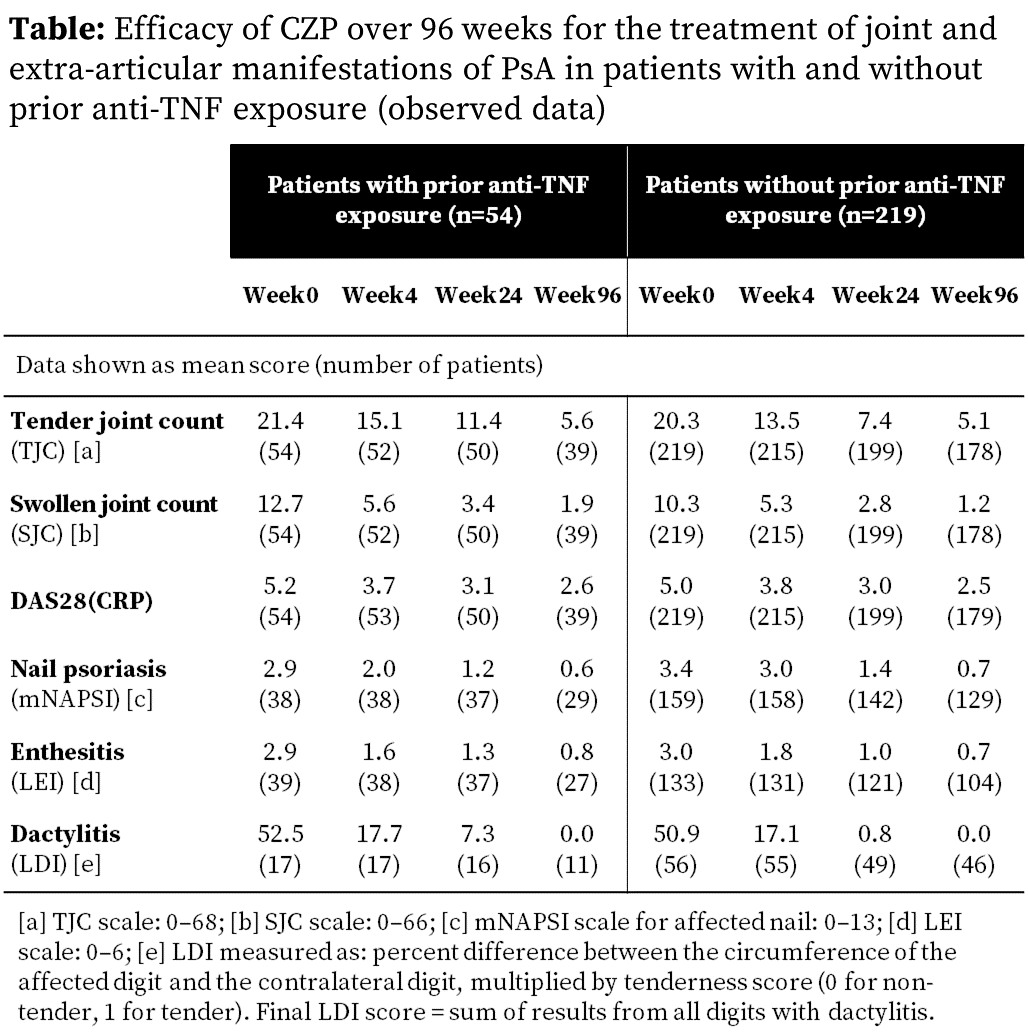
Results:
There were 409 pts randomized, 273 received CZP from
Wk0, of whom 54 (19.8%) had prior anti-TNF exposure. Of the 273 CZP-randomized
pts, 197 (72.2%) had BL nail psoriasis, 172 (63.0%) BL enthesitis and 73
(26.7%) BL dactylitis. BL scores for joints and EAMs were similar in pts with
and without prior anti-TNF exposure (Table).
By Wk4, CZP treatment was associated with rapid
improvements in all measures of joint disease activity, including TJC, SJC and
DAS28(CRP). Improvements in all outcomes continued through to Wk96 and were
very similar in pts with and without prior anti-TNF exposure (Table).
Similarly, improvements in nail psoriasis, enthesitis and dactylitis were seen
to Wk96 of CZP treatment in PsA pts regardless of their prior anti-TNF exposure
(Table). However, these analyses were limited by the low number of pts with
prior anti-TNF exposure.
Conclusion:
Improvements in joint outcome measures and EAMs of PsA
were observed over 96 wks of CZP treatment. These improvements were seen across
multiple joint outcome measures and in all EAMs assessed, including nail
psoriasis, enthesitis and dactylitis. Similar improvements were observed in pts
with and without prior anti-TNF exposure.
References:
1. Mease P. Arthritis Rheum 2014;66(S10):S237–8
2. Khraishi M. Ann Rheum Dis 2015;74(S2):353–4
3. Mease P. Ann Rheum Dis 2014;73(1):48–55
To cite this abstract in AMA style:
Gladman DD, Gottlieb AB, Hoepken B, Peterson L, FitzGerald O. Long-Term Improvements with Certolizumab Pegol in Joints and Extra-Articular Manifestations of Psoriatic Arthritis in Patients with and without Prior Anti-TNF Exposure [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/long-term-improvements-with-certolizumab-pegol-in-joints-and-extra-articular-manifestations-of-psoriatic-arthritis-in-patients-with-and-without-prior-anti-tnf-exposure/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-improvements-with-certolizumab-pegol-in-joints-and-extra-articular-manifestations-of-psoriatic-arthritis-in-patients-with-and-without-prior-anti-tnf-exposure/