Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase
inhibitor for the treatment of RA. A modified-release (MR) formulation to
provide a once-daily (QD) dosing alternative to the available 5 mg twice-daily
(BID) immediate-release (IR) formulation has been developed.1 The
objective was to determine the most relevant pharmacokinetic (PK) parameter
driving the clinical response of tofacitinib to inform clinical development of
the MR formulation.
Methods: Clinical efficacy data (DAS and ACR 20/50/70 responses) from 5 Phase 2
RA dose-ranging studies of IR 1-30 mg BID and IR 20 mg QD across ~1,500
patients were analyzed. Dose and exposure-response (E-R) models were developed
to characterize the time course of changes in DAS, including quantifying the
delay between concentration and response, and to compare the predictive
abilities of various PK parameters (total drug exposure as measured by area
under the concentration-time profile [AUC], and peak [Cmax]
and minimum [Cmin] plasma concentrations).
Non-clinical efficacy data from a dose-fractionation study of multiple IR QD
and BID doses using the murine collagen-induced
arthritis (mCIA) model were also analyzed to
delineate the predictive ability of PK parameters. The relationship between
tofacitinib exposure metrics and incidence of safety outcomes were explored
using linear logistic models.
Results: The E-R model yielded an equilibration half-life of 3.2 weeks for
changes in DAS, substantially longer than the PK half-life of tofacitinib (~3
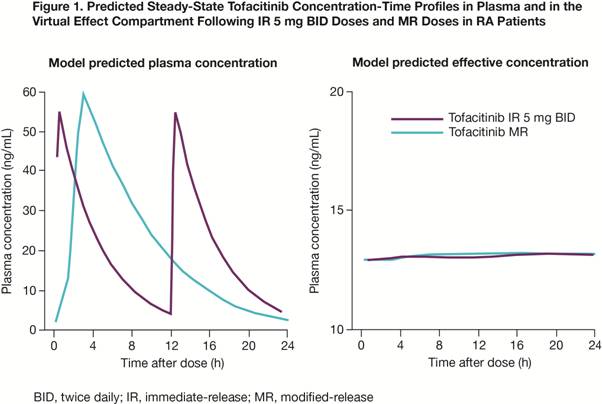
hours). Application of this model to plasma PK profiles of MR and IR yielded
predicted effective superimposable concentrations for
both regimens (Fig 1). In a dose-ranging Phase 2 study, analyses of the IR 20
mg QD dose (similar AUC to the IR 10 mg BID dose, but 86% lower Cmin and ~2-fold higher Cmax)
demonstrated similar efficacy to the IR 10 mg BID dose. DAS mean [standard
error] change from baseline was -1.72 [0.14] for IR 20 mg QD vs -1.82 [0.15] for IR 10 mg BID; ACR 20/50/70 rates were
56/36/24% for IR 20 mg QD vs 58/28/12% for IR 10 mg
BID, respectively, at Week 12. Statistical comparison of PK parameters favored
AUC as a better predictor (p<0.05) of DAS changes than Cmax
or Cmin, with little added value of Cmax or Cmin.
In the mCIA model, BID and QD E-R curves were well
aligned for AUC as the predictor, as demonstrated by concordance of
concentrations producing 50% of maximum response. Comparing the predictive
ability of AUC, Cmax, and Cmin for safety events of interest (eg serious infections) also supported AUC as the most
relevant PK parameter (data not shown).
Conclusion: Collectively, these analyses support AUC as the
relevant predictor of tofacitinib clinical response and inform clinical
development of an MR formulation of tofacitinib for QD dosing.
Reference
- Lamba M et al. Ann Rheum Dis 2015; 74(S2): 626.
To cite this abstract in AMA style:
Lamba M, Furst DE, Dikranian A, Dowty M, Hutmacher MM, Conrado D, Stock T, Nduaka C, Krishnaswami S. Evaluating Pharmacokinetic Predictors of Tofacitinib Clinical Response in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/evaluating-pharmacokinetic-predictors-of-tofacitinib-clinical-response-in-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-pharmacokinetic-predictors-of-tofacitinib-clinical-response-in-rheumatoid-arthritis/