Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient reported outcomes such as
morning stiffness are reported frequently in rheumatoid arthritis (RA)
patients. But little has been reported about the presence and the treatment of
it in patients who have achieved low disease activity (LDA) (1). The CAPRA-2 study
demonstrated previously absolute and relative reductions in morning stiffness
in RA patients on DMARDs and concomitantly treated with low dose
delayed-release (DR-) prednisone as compared to placebo/DMARDs over 12 weeks (2).
Herein we report the relative and absolute changes in morning stiffness from
the CAPRA-2 study in patients who achieved, and did not achieve, LDA.
Methods: RA patients with moderate disease on non-biologic
DMARDs previously randomized to receive DR-prednisone or placebo were evaluated
at baseline, 2, 6, and 12 weeks for relative and absolute changes in morning
stiffness and LDA status (DAS28 ≤3.2).
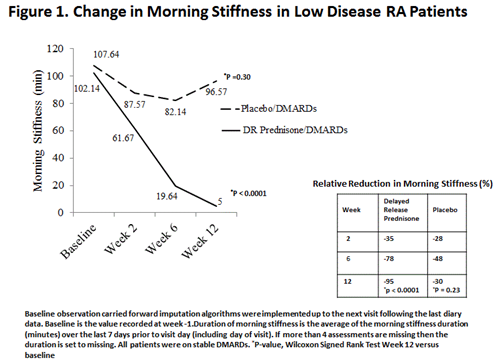
Results: Patients who attained LDA at 12 weeks had similar demographics
at the beginning of the trial to those who did not achieve LDA. By week 12,
patients who received DR-prednisone and attained LDA (N=62) had significantly
higher absolute (p<0.05) and relative reductions (p<0.001) in morning
stiffness as compared to patients who received DR-prednisone and did not reach
LDA (N=153). There were no differences in the placebo treated LDA (N=17) and
non-LDA (N=90) groups. Of the patients attaining LDA status the DR-prednisone
group had longer duration of disease, greater severity of morning stiffness and
greater pain upon awakening, but similar DAS scores, at baseline compared to
the placebo group. Despite this, there were significant differences from
baseline found in both absolute (p<0.0001) and relative reductions
(p<0.0001) in morning stiffness in those treated with DR-prednisone (n=62)
which was not observed with placebo patients reaching LDA (n=16). (Figure 1)
Conclusion: Attainment of LDA is not accompanied by
decreasing morning stiffness in patients on DMARD monotherapy but is with DR
prednisone/DMARDs, indicating the potential lack of construct validity between
the two outcomes in the absence of glucocorticoid therapy. Glucocorticoid/DMARD
treatment had a profound suppressing effect (-95%) on morning stiffness in
patients who reached low disease activity. It is unknown whether this construct
validity exists with other commonly used therapies in RA.
References:
(1) van
Tuyl, et al. BMC Musculoskeletal Disorders 2014;15:28-33.
(2) Buttgereit,
et al. Ann Rheum Dis 2013;72:204–210.
To cite this abstract in AMA style:
Alten R, Holt RJ, Kent JD, Buttgereit F. Analysis of Morning Stiffness Response in Rheumatoid Arthritis Patients with Low Disease Activity Receiving Delayed-Release Prednisone Plus Dmards As Compared to Placebo Plus Dmards [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/analysis-of-morning-stiffness-response-in-rheumatoid-arthritis-patients-with-low-disease-activity-receiving-delayed-release-prednisone-plus-dmards-as-compared-to-placebo-plus-dmards/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-morning-stiffness-response-in-rheumatoid-arthritis-patients-with-low-disease-activity-receiving-delayed-release-prednisone-plus-dmards-as-compared-to-placebo-plus-dmards/