Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Treat to
target (TTT) is a recommended paradigm in the management of rheumatoid
arthritis (RA). However, various data sources suggest that TTT is implemented
in only a minority of routine care settings in the US. We designed a cluster-randomized
controlled trial of a Learning Collaborative (LC) to facilitate uptake of TTT.
Methods: We recruited 11
rheumatology practices from across the US using sampling. They were randomized
into two groups: one is receiving the LC intervention in Phase 1 (months 1-9) and
the second formed a wait-list control group to receive the intervention in
Phase 2 (months 10-18). The sites were permitted to choose a disease activity
index from among 6 approved by an ACR committee. The LC intervention is a modified
version of the Institute for Healthcare Improvement’s Breakthrough Series
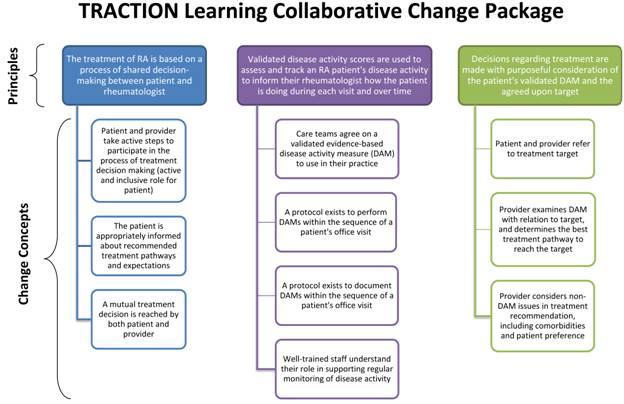
Collaborative method, using the Model for Improvement, a Change Package (see
Figure), and monthly metrics. Phase 1 intervention practices were brought
together for one face-to-face learning session. Continuous collaboration and
process improvement has been facilitated by several means: monthly webinars to
share results of Plan-Do-Study-Act (PDSA) cycles; a web-based tool for sites to
share aggregated site-level data and PDSA results; and monthly improvement
metrics collected at each practice. The wait-list control group sites have had
no TTT interventions during Phase 1.
The primary trial
outcome is degree of uptake of (“adherence with”) TTT as measured by chart
review of select patients within participating sites, comparing the differences
in adherence from baseline to end of Phase 1 between patients in intervention
and control practices. Specifically, the chart reviews measures 4 aspects of
TTT: evidence of shared decision-making, description of a treatment target,
measurement of disease activity, and response to disease activity (i.e., change
in treatment if not at target or reason for no change). Secondary outcomes
include: resource use, adverse events, disease activity, and sustained
adherence with TTT during Phase 2.
Results: Phase 1 will finish in
October 2015. All intervention sites have remained engaged in the LC with a
total of 38 providers (31 physicians, 6 nurses and 1 pharmacist) participating.
The primary trial outcome measures will be collected by the study team through
medical record review at completion of Phase 1.
Conclusion: If this LC is an
effective means for improving uptake of TTT in rheumatology practices, this
process could serve as a way of disseminating TTT more widely. As well, LCs
may be considered for other rheumatology quality improvement efforts.
To cite this abstract in AMA style:
Solomon DH, Lee S, Zak A, Agosti J, Bitton A, Fraenkel L, Harrold L, Losina E, Lu B, Pincus T, Smolen JS, Katz JN. Improving Adherence with Treat to Target in Rheumatoid Arthritis through a Learning Collaborative: Rationale and Design of the Traction Trial [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/improving-adherence-with-treat-to-target-in-rheumatoid-arthritis-through-a-learning-collaborative-rationale-and-design-of-the-traction-trial/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-adherence-with-treat-to-target-in-rheumatoid-arthritis-through-a-learning-collaborative-rationale-and-design-of-the-traction-trial/