Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The presence of aPL has been
associated with pregnancy complications, but the evolution of aPL titers during
pregnancy in aPL-positive patients and the utility of repeated testing during
pregnancy are unclear. The primary objective of the study was to evaluate
whether titers of aCL and anti-β2 Glycoprotein-1 antibodies (aβ2GP1) vary
during pregnancy. The second objective was to determine if aPL
variation was associated with pregnancy outcomes.
Methods:
Data were collected from the aPL-positive patients included
in PROMISSE Study, a multicenter, prospective, observational study of pregnancy
outcome in women with aPL and/or SLE. Demographic and
clinical data were collected at screening and patients followed monthly until
delivery. aPL were tested in a core laboratory at
screening [<18 weeks of gestation (WG)], 2nd trimester (20-23 WG),
3rd trimester (32-35 WG) and 12 weeks post partum (PP). Patients were
considered aPL-positive if aCL and/or aβ2GP1 was ³ 40IU IgG or IgM and/or LA was positive in two determinations
with at least one during pregnancy. Adverse pregnancy outcome (APO) was
defined as one or more of the following: fetal death after 12 WG; neonatal
death; delivery prior to 36 WG due to preeclampsia or placental insufficiency; or
SGA<5th percentile.
Results:
One hundred and fifty-eight patients fulfilled
inclusion criteria. Mean age was 31.9 ± 4.6 years, 84% were white, 57% had
clinical APS, and 36% had SLE. During pregnancy, 28% were treated with hydroxychloroquinine,
11% steroids, 66% heparin, and 71% aspirin. At screening, 58% were IgG aCL
positive, 18% IgM aCL positive, 30% aβ2GP1 IgG positive, 15% IgM
aβ2GP1 positive, and 52% were LA positive. APO occurred in 30% of the
patients.
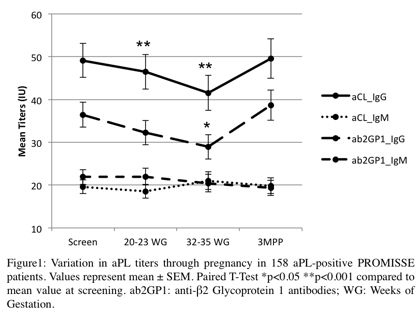
Description of mean aPL titer
variation is shown Figure 1. aPL IgG titers decreased
through pregnancy, and the mean aCL IgG titer was significantly lower during 2nd
and 3rd trimesters compared to screening, but remained in
the high positive range. Mean aβ2GP1 IgG titer was significantly lower during
3rd trimester. 80% of APO occurred during the 2nd
trimester when IgG titers began to fall. No significant variation in aCL or
aβ2GP1 IgM was observed through pregnancy, and titers remained low.
Of note, aPL IgG titers, as
well as magnitude of change in titer over the course of pregnancy, were comparable
between the patients with or without APO.
Conclusion:
In
a large, prospective cohort of aPL-positive pregnant women, aCL and aβ2GP1 IgG titers decreased modestly
in the course of pregnancy. Potential mechanisms for this decrease include dilutional effect, targeting of aPL
to placenta, or decreased autoantibody production. Changes were modest, not
clinically meaningful, and not associated with pregnancy outcomes. Our findings suggest that it is not
necessary to repeat
aPL testing through pregnancy.
To cite this abstract in AMA style:
Yelnik C, Porter F, Branch WD, Buyon JP, Guerra M, Laskin C, Lockshin M, Petri M, Merrill JT, Sammaritano LR, Stephenson MD, Kim MY, Salmon JE. Small but Clinically Insignificant Decreases in Antiphospholipid Antibody Titers Occur in aPL-Positive Patients during Pregnancy [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/small-but-clinically-insignificant-decreases-in-antiphospholipid-antibody-titers-occur-in-apl-positive-patients-during-pregnancy/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/small-but-clinically-insignificant-decreases-in-antiphospholipid-antibody-titers-occur-in-apl-positive-patients-during-pregnancy/