Session Information
Date: Monday, November 9, 2015
Title: Health Services Research II: Rheumatoid Arthritis Treatment and Healthcare Utilization
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Several trials suggest that triple
therapy with non-biologic disease-modifying antirheumatic drugs (ttDMARD) has
similar efficacy compared to biologic DMARDs (bDMARD) for patients with RA.
However, data on the use of ttDMARD in typical clinical practice and factors
associated with intensification to ttDMARD have not been thoroughly examined.
We evaluated progression to ttDMARD or bDMARD use after initial non-biologic
DMARD (nbDMARD) prescription among patients with RA.
Methods: We used medical and prescription
claims data from a large US commercial insurance program to evaluate ttDMARD
use in RA between 1/1/2009 and 6/30/2014. Patients with a visit for RA and
initial nbDMARD prescription were included after 180 days of continuous eligibility.
ttDMARD use was defined as prescriptions for methotrexate, sulfasalazine, and
hydroxychloroquine within 60 days. We calculated frequencies and rates for 6-month
periods for the intensification to ttDMARD or bDMARD after initial nbDMARD
prescription. We evaluated temporal, geographic, sociodemographic, clinical,
and healthcare utilization factors as possible correlates of intensification to
ttDMARD after initial nbDMARD using Cox regression models to estimate hazard
ratios (HR), 95% confidence intervals (CI), adjusting for potential confounders.
Results: We analyzed 24,576 patients with
initial nbDMARD prescription for RA during the study period. In this sample,
78% were female and mean age was 50.3 (SD 12.3) years. Methotrexate,
sulfasalazine, or hydroxychloroquine were initially prescribed for 21,584 (88%)
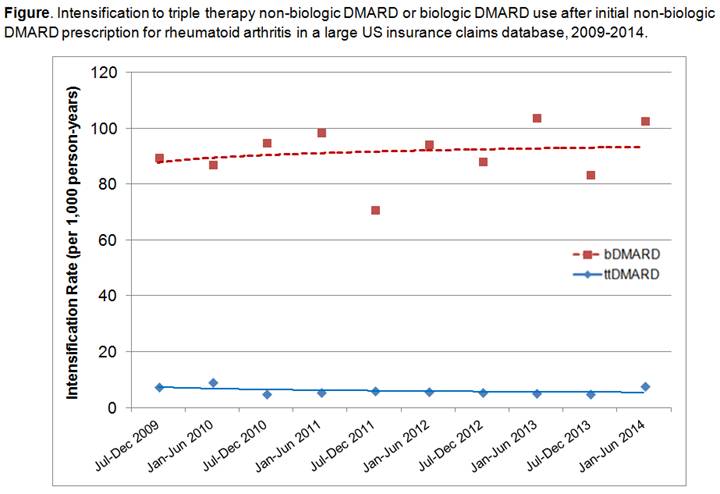
patients. During the entire 66-month study period, 2,739 (11.1%) intensified
treatment to bDMARD compared to 181 (0.7%) who intensified to ttDMARD (see Figure).
There was no increase in ttDMARD use over the study period. US geographic area
was associated with intensification to ttDMARD: West (HR 1.82, 95% CI 1.14-2.88),
South (HR 1.57, 95% CI 1.01-2.43), and Midwest (HR 1.78, 95% CI 0.99-3.20) compared
to Northeast. Glucocorticoid use (HR 2.09, 95% CI 1.52-2.88) and nonsteroidal
anti-inflammatory drug (NSAID) use (HR 1.62, 95% CI 1.19-2.21) prior to cohort
entry date were significantly associated with subsequent ttDMARD
intensification. Age, sex, year of entry into the cohort, median residence income,
comorbidities, history of serious infection, rheumatologist appointment, and healthcare
utilization factors were not associated with intensification to ttDMARD after
initial nbDMARD prescription.
Conclusion: Despite reports during our study
period suggesting equivalent efficacy of ttDMARD and bDMARD for RA, the use of
ttDMARD after initial nbDMARD was infrequent and did not increase over time in
this large nationwide study. Only 0.7% of RA patients were prescribed ttDMARD,
despite 88% initially being prescribed methotrexate, sulfasalazine, or
hydroxychloroquine. Further research investigating the use of ttDMARD for RA is
warranted.
To cite this abstract in AMA style:
Sparks JA, Krumme AA, Matlin OS, Brill G, Shrank WH, Choudhry NK, Solomon DH. Intensification to Triple Therapy Non-Biologic Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis in the United States from 2009 to 2014 [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/intensification-to-triple-therapy-non-biologic-disease-modifying-antirheumatic-drugs-for-rheumatoid-arthritis-in-the-united-states-from-2009-to-2014/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intensification-to-triple-therapy-non-biologic-disease-modifying-antirheumatic-drugs-for-rheumatoid-arthritis-in-the-united-states-from-2009-to-2014/