Session Information
Date: Monday, November 9, 2015
Title: Rheumatoid Arthritis-Small Molecules, Biologics and Gene Therapy III: Biosimilars
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: CT-P10
is a biosimilar candidate of innovator rituximab (RTX). PK profile and clinical
data up to week 24 has been reported at ACR 20131. Additional safety
and efficacy were evaluated to confirm similarity between CT-P10 and RTX up to 72
weeks (NCT01534884).
Methods: Patients
with active RA (1987 ACR criteria) and intolerant or unresponsive to anti TNF-a
blocker were randomized 2:1 to receive 2 infusions (1,000 mg, IV each) of
either CT-P10 or RTX, at a 2-week interval, in combination with methotrexate
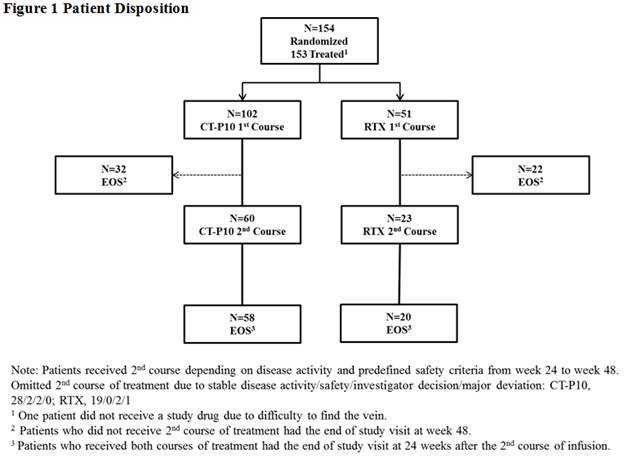
(MTX) and folic acid. The second course of treatment was initiated between
weeks 24 ~ 48 based on disease activity and predefined safety criteria. The end
of study visit for patients who did not receive the second course of treatment
was conducted at week 48 (Figure 1). Efficacy was analyzed in per-protocol
population.
Results: Of
154 patients randomized at baseline, 132 (85.7%) patients completed the study. The
efficacy in terms of DAS28 changes and overall safety were comparable between CT-P10
and RTX treatment groups. The mean decreases from baseline in DAS28 at Week 24
after 1st and 2nd courses of treatment were similar between
CT-P10 and RTX treatment groups (Table 1). The proportion of patients experienced
at least one adverse event (AE), serious AE, infusion related reaction,
infection, malignancy or lymphoma and discontinuation due to AE was similar
between CT-P10 and RTX treatment groups (Table 2). Cervix carcinoma stage 0 was
reported in a patient from RTX group, and no deaths were reported. B-cell
kinetics between the CT‑P10 and RTX treatment groups were similar at all time
points. The mean changes from baseline in IgM, IgG, and IgA were small, and
there were no notable differences between the CT-P10 and RTX treatment groups.
Table
1
Efficacy of CT-P10 and Innovator Rituximab by DAS28 Changes
|
CT-P10 |
RTX |
|
|
DAS28-CRP, Mean ± SD (n) |
||
|
Baseline |
6.0 ± 0.9 (100) |
6.0 ± 0.8 (47) |
|
Changes at Week 24 after 1st Course |
̶ 1.9 ± 1.2 (95) |
̶ 2.0 ± 1.5 (43) |
|
Changes at Week 24 after 2nd Course |
̶ 2.4 ± 1.3 (58) |
̶ 2.0 ± 1.2 (20) |
|
DAS28-ESR, Mean ± SD (n) |
||
|
Baseline |
6.8 ± 0.8 (100) |
6.7 ± 0.8 (47) |
|
Changes at Week 24 after 1st Course |
̶ 2.1 ± 1.2 (95) |
̶ 2.1 ± 1.5 (43) |
|
Changes at Week 24 after 2nd Course |
̶ 2.5 ± 1.3 (58) |
̶ 2.0 ± 1.2 (20) |
|
RTX, innovator rituximab; DAS28, disease activity score in 28 joints; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation |
||
Table 2
Safety Summary of CT-P10 and Innovator Rituximab
|
CT-P10 N=102 |
RTX N=51 |
|
|
Number of Patients (%) with at Least One |
||
|
AE |
73 (71.6) |
43 (84.3) |
|
Serious AE |
14 (13.7) |
7 (13.7) |
|
Infusion-related reaction |
20 (19.6) |
10 (19.6) |
|
Infection |
39 (38.2) |
21 (41.2) |
|
Malignancy |
0 |
1 (2.0) |
|
Discontinuation due to AEs |
6 (5.9) |
4 (7.8) |
RTX, innovator rituximab; AE, adverse events
Conclusion: CT-P10,
a biosimilar candidate of RTX, showed highly similar efficacy and comparable safety
profiles to RTX up to 72 weeks.
Reference
1 Yoo DH, et al.
Arthritis Rheum 2013; 65 (Suppl 10): S736
To cite this abstract in AMA style:
Yoo DH, Park W, Suh CH, Shim SC, Jeka S, Cons Molina F, Hrycaj P, Spieler W, Wiland P, Brzezicki J, Lee EY, Medina-Rodriguez FG, Shesternya P, Radominski S, Stanislav M, Kovalenko V, Sheen D, Myasoutova L, Lim MJ, Choe JY, Kwon TS, Lee SJ. Efficacy and Safety of Rituximab Biosimilar Candidate (CT-P10) and Innovator Rituximab in Patients with Rheumatoid Arthritis: Results from Phase I Randomized Controlled Trial over 72 Weeks [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-rituximab-biosimilar-candidate-ct-p10-and-innovator-rituximab-in-patients-with-rheumatoid-arthritis-results-from-phase-i-randomized-controlled-trial-over-72-weeks/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-rituximab-biosimilar-candidate-ct-p10-and-innovator-rituximab-in-patients-with-rheumatoid-arthritis-results-from-phase-i-randomized-controlled-trial-over-72-weeks/