Session Information
Date: Monday, November 9, 2015
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Persistence
and compliance with oral osteoporosis therapies are generally suboptimal;
however, limited persistence data exist for newer injectable therapies that
have become available in recent years. The objective of this study is to
examine persistence and compliance of osteoporosis therapies using real-world
data from a large national health plan in the United States (US).
Methods: Women
affiliated with a large US national health plan who newly initiated an osteoporosis
medication (alendronate, denosumab, ibandronate, raloxifene, risedronate, or
teriparatide) between January 2012 and December 2012 were identified using the
Optum Research Database. The index date was the first qualifying claim date.
Patients were required to be ≥ 50 years of age at index and
have ≥
12
months of pre-index and ≥ 12 months of post-index
continuous enrollment in the health plan. Patients
with Paget’s disease of the bone, osteogenesis imperfecta, hypercalcemia,
malignant cancer and metastasis, HIV, and patients receiving preventive
treatment for risk of breast cancer were excluded. Persistence
(continuous use of index therapy with no gap > 60 days) and compliance
(proportion of days covered by therapy ≥ 80%) were assessed during the
12-month follow-up period. Multivariable logistic regression models were used
to estimate and compare persistence and compliance for the therapies of interest,
adjusting for demographic and clinical characteristics (reference = group with
highest persistence/compliance).
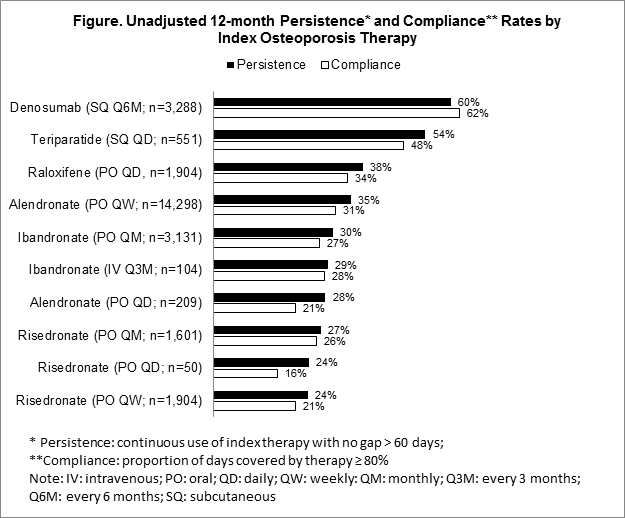
Results: A
total of 27,040 patients were eligible and included in the study (mean [SD] age:
67.2 [10.1] years). 12-month persistence was highest for denosumab
(subcutaneous every 6 months) at 59.9% and lowest for risedronate (oral weekly)
at 23.8%; 12-month compliance was highest for denosumab at 62.2% and lowest for
risedronate (oral daily) at 16.0% (Figure). The multivariable logistic
regressions showed that the odds of being persistent and compliant across
treatments favored denosumab (odds ratios from 1.4 to 4.9, p<0.001 for
persistence; 1.9 to 8.8, p<0.001 for compliance).
Conclusion: In
a large US national health plan, persistence and compliance over 12 months were
higher among patients initiating denosumab compared to those initiating other
osteoporosis therapies.
To cite this abstract in AMA style:
Chastek B, Cheng LI, White JC, Spangler L, Mehta D, Barron R. Persistence with Osteoporosis Therapies in Postmenopausal Women in a Large US National Health Plan [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/persistence-with-osteoporosis-therapies-in-postmenopausal-women-in-a-large-us-national-health-plan/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-with-osteoporosis-therapies-in-postmenopausal-women-in-a-large-us-national-health-plan/