Session Information
Date: Monday, November 9, 2015
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Development
of tumor necrosis factor (TNF) inhibitors has been a major advance in treatment
of systemic inflammatory diseases, such as rheumatoid arthritis. While TNF
inhibitors are highly effective in controlling systemic inflammation, their
high cost limits their use in developing countries and raises concerns in the
U.S. as well. The FDA is currently considering an application for a biosimilar
version of infliximab, which has been available in South Korea since November
2012.
Methods:
Using
the medical claims data (4/2009-3/2014) of the Korean Health Insurance Review
and Assessment Service database, which includes the entire Korean population,
we assessed the uptake of biosimilar infliximab. A segmented linear regression
was used to examine utilization patterns of infliximab (the branded and
biosimilar) and other branded TNF inhibitors (adalimumab and etanercept) before
and after the introduction of biosimilar infliximab. The model included the
number of claims by drug each month as the outcome variable, as well as an intercept
and two slope terms that described the trend in use of TNF inhibitors per month
before and after the introduction of biosimilar infliximab in November 2012.
Results:
We
identified a total of 20,976 patients with mean age of 44 (SD 16) years who
used adalimumab, etanercept, infliximab, or biosimilar infliximab during the
study period. Since its introduction, there were 983 users of biosimilar infliximab.
Among all the claims for any infliximab version, the proportion of biosimilar
infliximab claims increased to 19% through 3/2014. The use of all TNF
inhibitors increased significantly with the number of claims approximately
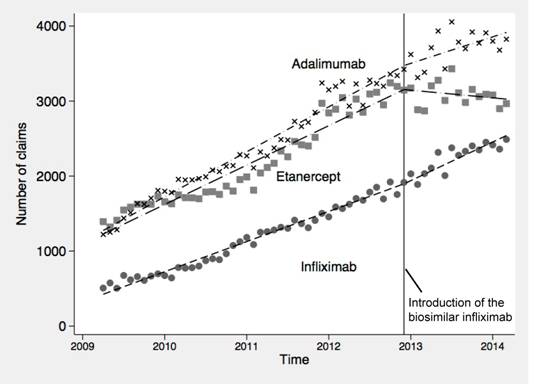
tripling from 3,117 in April 2009 to 9,278 in March 2014. Prior to introduction
of biosimilar infliximab, each month there were 33 (95%CI, 32-35) more
infliximab claims, 44 (95%CI, 40-48) more etanercept claims, and 50 (95%CI, 47-53)
more adalimumab claims (Figure). After the introduction of biosimilar
infliximab, there was a significant change in the slopes with an additional
increase in the use of both branded and biosimilar infliximab (9 claims/month,
95%CI, 2-17) and a decrease in the use of etanercept (-52 claims/month, 95%CI, -66
to -38) and adalimumab (-21 claims/month, 95%CI, -35 to -6).
Conclusion:
During
the 15 months since its introduction in South Korea, one-fifth of all
infliximab claims were for the biosimilar. Our results show that introduction
of biosimilar infliximab may affect the use of other TNF inhibitors. These
results suggest that an approved biosimilar infliximab product could have a
major impact in the U.S., where about 40% of rheumatoid arthritis patients get
treated with a biologic drug.
Figure. Trends in use of all
TNF-α inhibitors before
and after market introduction of biosimilar infliximab
Infliximab after the red
vertical line includes both the branded and biosimilar.
To cite this abstract in AMA style:
Kim SC, Choi NK, Lee J, Kwon KE, Eddings W, Sung YK, Song HJ, Kesselheim AK, Solomon DH. Uptake of the First Biosimilar Infliximab Since Its Approval in South Korea [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/uptake-of-the-first-biosimilar-infliximab-since-its-approval-in-south-korea/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/uptake-of-the-first-biosimilar-infliximab-since-its-approval-in-south-korea/