Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
The objective of this analysis was to examine persistence with intravenous (IV)
and subcutaneous (SC) anti-TNF therapies among rheumatoid arthritis (RA)
patients (pts) within the US CORRONA RA registry.
Methods: A
retrospective analysis from the CORRONA RA registry was used for analyses.
Adult RA pts (biologic-naïve and biologic-experienced) with moderate-to-severe
RA (CDAI>10) who initiated anti-TNF biologics within the CORRONA registry after
June 1, 2009 and had ≥6 months of follow up were eligible. Pts receiving IV
anti-TNF therapy were compared with those receiving SC anti-TNF therapy. Propensity
scores (PS) were generated to match IV anti-TNF initiators to SC anti-TNF
initiators, stratified by line of therapy. Variables used for PS matching were
based on characteristics with standardized differences of ≥0.1 including age,
gender, duration of RA, work status, smoking status, insurance, prednisone use,
monotherapy, prior serious infections, patient global, morning stiffness, year
of initiation, time from initiation to 6 month visit, and prior number of biologics
to control for population imbalances. Time-varying hazard ratios (HR) comparing
persistence in matched IV vs SC through 48 months were estimated and stratified
by line of therapy.
Results: A
total of 1544 pts were included (772 each for IV and SC therapy). In each
treatment group, 387 pts were bio-naïve, 220 had received 1 prior biologic, and
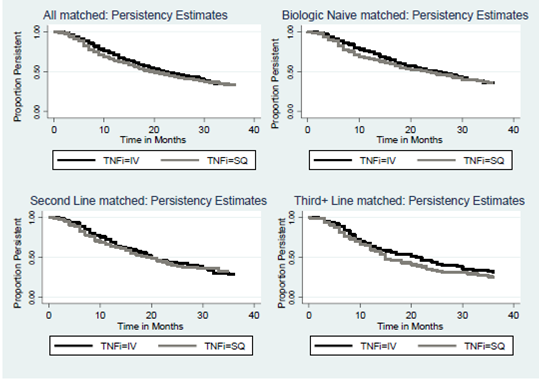
165 had received ≥2 prior biologics. Among all pts, significantly greater
proportions of patients receiving IV therapy remained on that agent compared
with pts receiving SC therapy (p=0.002) during the first 12 months (HR=0.76
[0.63-0.90]). No significant differences in persistence were observed from 24 –
48 months (HR=1.09 [0.88-1.35], p=0.45) (Figure 1). Similar results were
observed for pts who were biologic-naïve (0-12 mons: HR=0.69 [0.52-0.90],
p=0.007) and who had received 1 prior biologic (0-12mons: HR=0.80 [0.59-1.10],
p=0.17). For pts who had received ≥2 biologics, there was no significant
difference in persistence between patients receiving IV or SC anti-TNF therapy.
Conclusion:
Within the first 12 months of initiating anti-TNF therapy, pts receiving IV
therapy who were biologic-naïve or had received only one prior biologic were
more likely to remain on treatment compared with those receiving SC anti-TNF
agents. These results may have implications on cost savings, in the first year
of treatment, that need to be investigated in further studies.
Figure1.
Persistence estimates for matched populations.
To cite this abstract in AMA style:
Parenti D, Kafka S, Reed GW, Greenberg JD, DeHoratius R. Persistence Among Rheumatoid Arthritis Patients Initiating Intravenous or Subcutaneous Anti-Tumor Necrosis Factor Therapy in a Large US Registry Cohort [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/persistence-among-rheumatoid-arthritis-patients-initiating-intravenous-or-subcutaneous-anti-tumor-necrosis-factor-therapy-in-a-large-us-registry-cohort/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-among-rheumatoid-arthritis-patients-initiating-intravenous-or-subcutaneous-anti-tumor-necrosis-factor-therapy-in-a-large-us-registry-cohort/