Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In
patients (pts) with RA, the predictive value of baseline (BL) titers of anti-citrullinated protein antibodies (ACPA), a
known biomarker for RA and disease progression,1,2 on treatment
outcomes is not well understood. In this post
hoc analysis of the AMPLE study,3 we assessed the efficacy of SC abatacept (ABA) and adalimumab (ADA)
in pts with different BL levels of anti-cyclic citrullinated peptide 2 (CCP2) antibodies
(a surrogate for ACPA).
Methods: Pt samples were analyzed by anti-CCP2 immunoglobulin
(IgG) ELISA4 and efficacy outcomes were assessed in positive pts
divided into equal quartiles (Q) based on BL titer. Q1–Q4 represent increasing
titers of anti-CCP2 antibody. Efficacy outcomes analyzed by Q were: mean
change from BL in DAS28 (CRP) and HAQ-DI over time, and remission rates in
terms of CDAI, SDAI, and DAS28 [CRP] <2.6-defined remission. Mean change from BL was determined by analysis of covariance, with treatment and DAS28 (CRP)
stratification as factors, and BL values as a covariate. Data are also
presented for change from BL in DAS28 (CRP) over time in pts who were anti-CCP2 antibody negative at
BL.
were 97 pts per Q. The numbers of pts per treatment group in each Q were (ABA,
ADA): Q1=42, 55; Q2=51, 46; Q3=46, 51; Q4=46, 51. Overall BL
characteristics were generally comparable, with no discernible pattern across
Qs and treatment groups. For example, pts in ABA Q4 and ADA Q2 had numerically
higher DAS28 (CRP) but lower HAQ-DI scores than pts in other Qs. For ABA, mean improvements from BL in DAS28 (CRP) and HAQ-DI
were greater in the highest titer anti-CCP2 Q compared with the other Qs. The
95% CI of these measures for the highest and lowest titer Q did not overlap. There
was no apparent association between these efficacy measures and BL anti-CCP2 titer
in the ADA group. Adjusted mean change from
BL in DAS28 (CRP) over time by BL anti-CCP2 Q, including pts anti-CCP2 negative
at BL, is shown in the Figure. Reductions in DAS28 (CRP) and HAQ-DI by Year 2 were
generally comparable in Q1–3 in both ABA and ADA groups. Remission rates
across all indices were broadly similar in ABA and ADA groups in Q1–3, but were
numerically greater in Q4 compared with Q1–3 in the ABA group only.
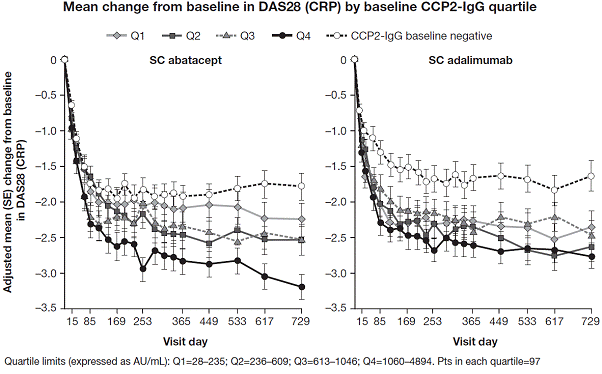
titer anti-CCP2 antibody at BL is correlated with better efficacy in pts from
the AMPLE study treated with abatacept, but not with adalimumab.5
1. Verpoort
KN, et al. Arthritis Rheum 2006;54:3799–808.
2. van der Woude
D, et al. Ann Rheum Dis 2010;69:1554–61.
3. Schiff M, et al. Ann
Rheum Dis 2014;73:86–9.
4. Anti-CCP2 ELISA, Euro Diagnostica. http://www.eurodiagnostica.com/upload/files/fileLibrary/ProductSheet_CCPlus_Screen120504.pdf.
Accessed 15 Jan 2015.
5. This abstract was first
presented at the EULAR Congress, 10–13 June 2015, Rome, Italy (AB0274) and
published in the corresponding supplement of Ann Rheum Dis.
To cite this abstract in AMA style:
Sokolove J, Schiff M, Fleischmann R, Weinblatt M, Connolly S, Johnsen A, Zhu J, Maldonado M, Patel S, Robinson W. Impact of Baseline Anti-Cyclic Citrullinated Peptide 2 Antibody Titer on Efficacy Outcomes Following Treatment with Subcutaneous Abatacept or Adalimumab [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/impact-of-baseline-anti-cyclic-citrullinated-peptide-2-antibody-titer-on-efficacy-outcomes-following-treatment-with-subcutaneous-abatacept-or-adalimumab/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-baseline-anti-cyclic-citrullinated-peptide-2-antibody-titer-on-efficacy-outcomes-following-treatment-with-subcutaneous-abatacept-or-adalimumab/
