Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept (ABA) is a
biological anti-rheumatic drug used in Rheumatoid Arthritis (RA). In TURKBIO,
Turkish Biologic Registry, data on patient characteristics, diagnosis, previous
treatment and outcomes of ABA have been collected since 2011. Drug retention is
a useful measure of effectiveness since it combines both clinical response and
tolerability. The objective of this study was to investigate the effect of
rheumatoid factor (RF) and Anti-Cyclic Citrullinated
Peptide (Anti-CCP) positivity on drug survival of ABA in clinical practice for
patients with RA.
Methods: By the end of May 2015, 266 patients received
intravenous and subcutaneous ABA from 5 participating centers of the TURKBIO
registry were included in the analysis. Demographic and clinical data including
age, sex, disease duration, drug retention rate and reasons for discontinuation
of drug were collected. Kaplan-Meier survival analysis was performed to
estimate the drug survival. Subgroups were compared by log-rank.
Results: Of the 266 patients receiving ABA, 83.8% were female
and median age was 55.0 years (range: 19.0-81.0). The median disease duration
was 9.0 years (range: 1.0 year-53.0 years). 72.6% of were treated with ABA as
first line biotherapy (n=193), 21.8% as second line (n=58) and 3.4% as third
line (n=9). The rate of RF positive patients was 54.1% and anti-CCP positive was
68.1% among the patients treated with ABA in the registry. Estimated drug
survival rates for ABA in 6th month, 1st year and 2nd
year were 76.1 %, 65.5% and 52.2% . Median drug survival
was 26 months. One-year drug survival of first line and >1st line
ABA treatment were 68.7% and 57.9%, respectively (p=0.084). There was a trend
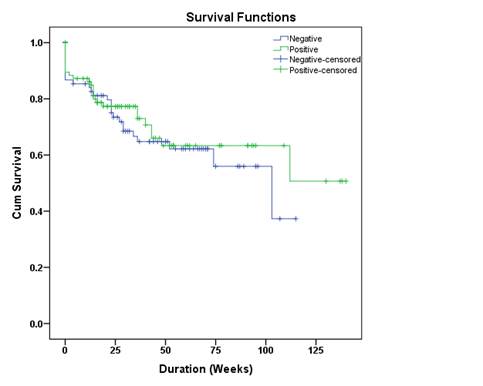
for a higher one year drug survival rate among anti-CCP (+) patients as compared
to those who were anti-CCP (-) (73.1% versus 56.4%; p=0.087), whereas one-year
drug survival rates were very similar for RF (+) and RF (-) patients at 63.4% and 64.8%, respectively (p=0.462) Drug
survival curves for RF (+)/(-) and Anti-CCP (+)/(-) groups are given in Figure
1 and 2, respectively.
Figure 1. Drug survival curve in RF (+) and (-) patients.
Figure 2.
Drug survival curve in anti-CCP (+) and (-) patients
Conclusion: One year drug survival rate of ABA among patients
enrolled in the TURKBIO registry is similar to those which has
been reported in some other European countries. One year drug survival rates
appear to be better among patients who are positive for anti-CCP, but not for
RF as compared to those who are negative for the corresponding antibodies,
which needs to be explored in future studies.
To cite this abstract in AMA style:
Ertenli I, Karadag O, Pehlivan Y, Dalkilic E, Onat AM, Kisacik B, Can G, Akar S, Capar S, Kalyoncu U, Oksuz MF, Tarhan EF, Akkoc N. The Effect of Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Positivity on Drug Survival of Abatacept in Patients with Rheumatoid Arthritis in Routine Care: The Results from Turkbio Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-rheumatoid-factor-and-anti-cyclic-citrullinated-peptide-positivity-on-drug-survival-of-abatacept-in-patients-with-rheumatoid-arthritis-in-routine-care-the-results-from-turkbio-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-rheumatoid-factor-and-anti-cyclic-citrullinated-peptide-positivity-on-drug-survival-of-abatacept-in-patients-with-rheumatoid-arthritis-in-routine-care-the-results-from-turkbio-registry/