Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The subcutaneous (SC) formulation
of abatacept (ABA) has been available in Canada since January 2014. Here we
report first year experience with SC ABA in Canadian RA patients (pts).
Canadian guidelines recommend the use of anti-TNFs or other mode of action biologics,
including abatacept, after inadequate response to DMARD.1 Furthermore,
the availability of an SC formulation of a biologic increases the treatment
options available to pts, particularly those wishing to self-administer their
therapy.
Methods: Canadian RA patients who accepted
treatment with abatacept (Orencia®) were enrolled in the Orencia® Response
Program (ORP) registry. RA pts treated with SC ABA in routine practice between
Jan – Dec 2014, were included in this analysis. Durability of treatment with SC
ABA was assessed using Kaplan-Meier survival analysis.
Results: As of December 2014, a total of 1152 pts received
SC ABA. Out of these, 698 (61%) received SC ABA as the first or second line
biologic therapy and 454 (39%) as third or fourth line. The median age of pts
prescribed SC ABA was 60 years (range 16-89); 79.3% were females; and the
median time since diagnosis for the entire cohort was 11 years. 72% of pts had
severe and 23% had moderate disease. The majority of pts received their treatment
at home (68%), followed by rheumatology (12%) or ORP clinic (7%). Only 12 (1%)
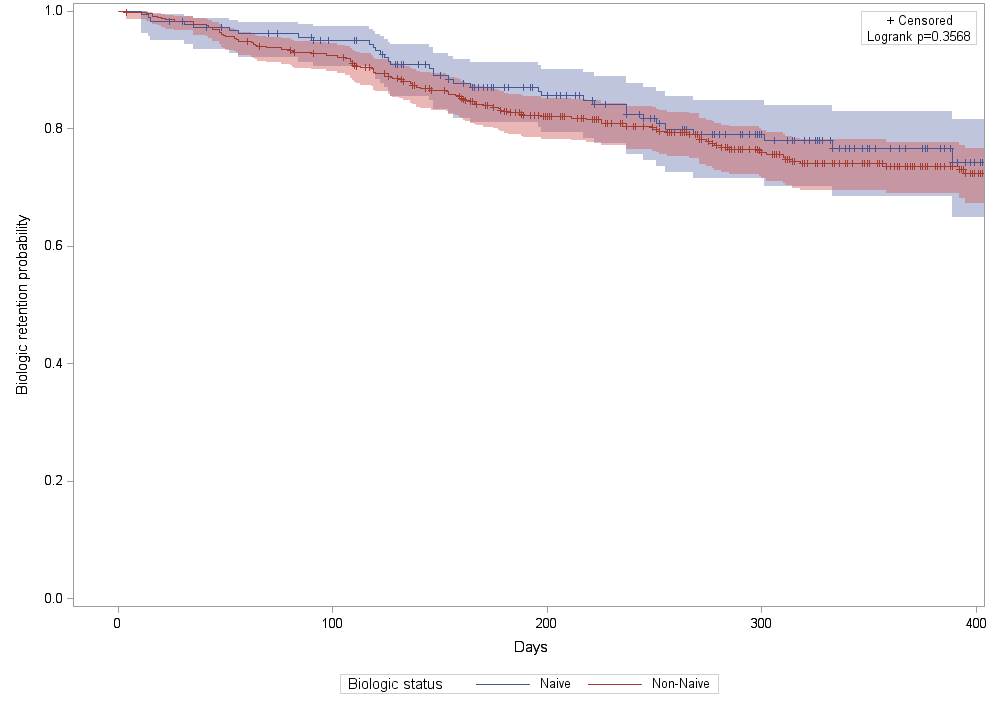
of pts received the treatment at a doctor’s office. 12-month persistence rates
were similar in biologic naïve and non-naïve patients: 76.7% (SD=3.7%) for pts
receiving SC ABA first line (biologic naïve) and 73.6% (SD=2.2%) for biologic
experienced (non-naïve pts), Figure 1.
Conclusion: This data demonstrate that SC ABA can be
used early in the course of the disease (first or second line biologic therapy)
as well as in patients who fail several previous lines of biologics. Treatment
with abatacept resulted in high 1-year persistency rates in both biologic naïve
pts as well as those who failed one or more previous lines of biologic therapy.
1Bykerk VP, et al. J Rheumatol.
2012;39(8):1559-1582.
Figure 1. First year persistency rates in patients receiving
SC abatacept
To cite this abstract in AMA style:
Haraoui B, Coupal L, Day R, Budry L, Chalabi Y. First Year Canadian Experience with Subcutaneous Abatacept in Routine Practice for the Treatment of Patients with Rheumatoid Arthritis: Data from the Orencia Response Program (ORP) Network [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/first-year-canadian-experience-with-subcutaneous-abatacept-in-routine-practice-for-the-treatment-of-patients-with-rheumatoid-arthritis-data-from-the-orencia-response-program-orp-network/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/first-year-canadian-experience-with-subcutaneous-abatacept-in-routine-practice-for-the-treatment-of-patients-with-rheumatoid-arthritis-data-from-the-orencia-response-program-orp-network/