Session Information
Date: Sunday, November 8, 2015
Title: Osteoporosis and Metabolic Bone Disease - Clinical Aspects and Pathogenesis Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although a higher risk of osteonecrosis of the jaw (ONJ) has been associated with antiresportive treatment based on spontaneous reports and observational studies, no study has systematically assessed the ONJ risk associated with Prolia (denosumab 60 mg). This study estimates the incidence rate (IR) of ONJ in users of Prolia or BP for postmenopausal osteoporosis (PMO) in administrative databases.
Methods: Using administrative data from US Medicare, Optum and Scandinavian national medical registries (Denmark, Norway and Sweden), postmenopausal women with a diagnosis of osteoporosis or osteoporotic fracture, or osteoporosis medications were identified from May 2010 to Dec 2011 (Medicare and Scandinavian national registries) or Mar 2013 (Optum). Women were followed for incident event of potential ONJ defined by ICD codes. Prolia and BP (oral and IV) exposure was updated in a time-varying manner and characterized “as treated”, with and without a 1-year extension following the on-treatment period defined as the days supplied + 60 days. The age-standardized IR of ONJ was computed for each exposure cohort.
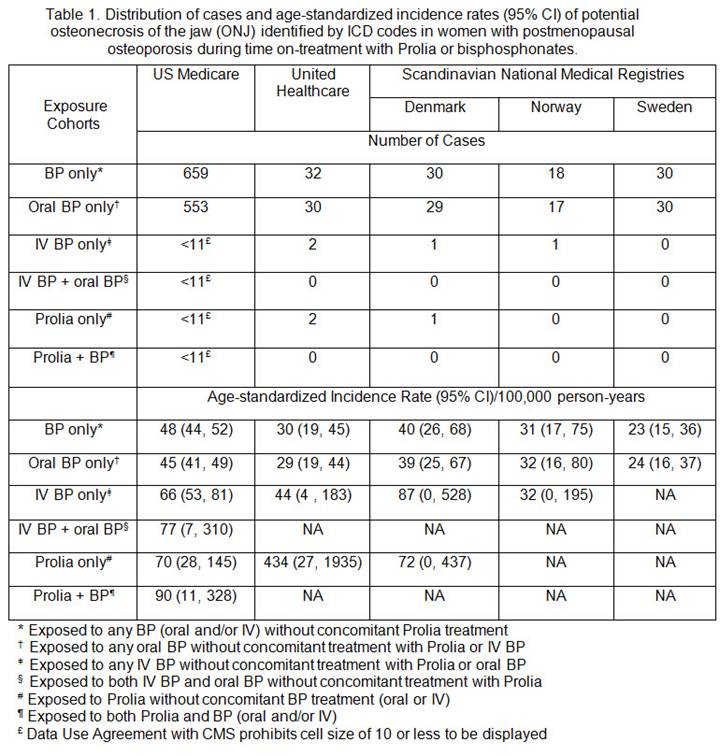
Results: A total of 2,561,119 women with PMO were identified from Medicare (1,995,915), Optum (193,003), Denmark (106,691), Norway (95,628) and Sweden (169,882). The IR (95% CI) per 100,000 person-years of ONJ in women with PMO was 40 (38–42) in Medicare, 27 (20–34) in Optum, 37 (27–50) in Denmark, 17 (11–26) in Norway and 25 (17–38) in Sweden. In Medicare, when exposure cohorts were classified only by the on-treatment period, the IR (95% CI) was 70 (28–145) in the Prolia only cohort and 90 (11–328) in the Prolia + BP cohort, and ranged from 45 (41–49) to 77 (7–310) in cohorts exposed to BP only (Table 1). When exposure cohorts were classified by on-treatment plus 1-year post-treatment period, the IR (95% CI) was numerically lower in the Prolia only cohort [59 (11–180)] and the Prolia + BP cohort [76 (30–160)], and remained similar in cohorts exposed to BP only [ranging from 45 (42–48) to 84 (53–125)] relative to the corresponding on-treatment only cohorts (Table 2). The number of ONJ cases in the other data systems was too low (0–2) in Prolia users to allow a robust estimation of the IR.
Conclusion: The descriptive analysis based on Medicare suggested the IR of potential ONJ was low in the PMO population and did not differ substantially by treatment. The number of cases in other data systems was low and precluded meaningful interpretation of the ONJ IR, especially in Prolia recipients. To confirm these results, further analysis based on medically confirmed cases and adjustment for confounders is warranted.
To cite this abstract in AMA style:
Xue F, Wagman RB, Yue S, Smith S, Arora T, Curtis JR, Ehrenstein V, Sørensen HT, Tell G, Kieler H, Wang FT, Dore DD, Sprafka JM. Incidence Rate of Potential Osteonecrosis of the Jaw Among Women with Postmenopausal Osteoporosis Treated with Prolia or Bisphosphonates [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/incidence-rate-of-potential-osteonecrosis-of-the-jaw-among-women-with-postmenopausal-osteoporosis-treated-with-prolia-or-bisphosphonates/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-rate-of-potential-osteonecrosis-of-the-jaw-among-women-with-postmenopausal-osteoporosis-treated-with-prolia-or-bisphosphonates/