Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Certolizumab pegol (CZP) is an Fc-free, PEGylated, anti-TNF approved in the US for the treatment of Crohn’s disease (CD) and rheumatoid arthritis (RA). Pre-clinical and clinical data suggest a lack of active neonatal Fc receptor-dependent placental transfer of CZP [1, 2]. There are few reports of pregnancy outcomes following exposure to CZP to date. This work provides additional information regarding the primary pregnancy outcomes in women exposed to CZP.
Methods:
The global CZP safety database was searched for all medically confirmed cases of pregnancy through March 6, 2012. The proportion of live births, spontaneous miscarriages, and elective terminations for women directly exposed to CZP before or during confirmed pregnancy were compared to those expected for the general US population of pregnant women.
Results:
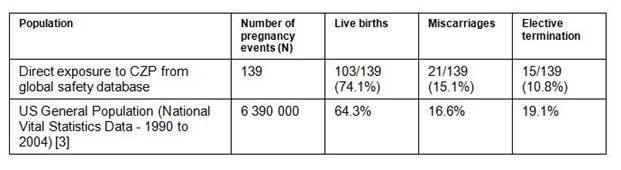
Of 294 reported pregnancy events, 152 had known outcomes, 89 had unknown outcomes and 53 were ongoing. Of the 152 events with known outcomes, 139 were cases in which the mother had direct exposure to CZP, with 57 from the clinical trial program and 82 from post-marketing reports. The remaining 13 were cases with the father exposed to CZP resulting in 10 live births, 2 miscarriages and 1 elective termination. Of the 139 direct exposure cases with known outcomes, the underlying conditions were CD (N=107), RA (N=17) and healthy subjects (N=2) with 13 cases classified as other or having missing data. 91 of 139 cases were from the US. 103 of 139 pregnancies resulted in live births (see table) and the median gestational age was 38.3 weeks (data available for 40 births). 21 pregnancies ended in spontaneous miscarriage. 15 pregnancies resulted in elective termination. These results are similar to those reported in the general population in the US (see table). In 103 live births there were 2 reported cases of congenital disorder (Rate in the US general population is 3% [4]); 1 baby had mild, unilateral hydronephrosis on antenatal ultrasound and was described as healthy upon birth. The other baby had vesicoureteric reflux.
Conclusion:
Currently available data from 139 pregnant women exposed to CZP, report outcomes consistent with the US National Vital Statistics data. Additional data from larger numbers of pregnant women exposed to CZP are required to validate acceptable safety and tolerability of CZP in pregnancy.
References:
1. Wakefield I et al. Toxicol Sci 2011;122(1):170-6. 2. Wolf D and Mahadevan U. Arthritis Rheum 2010;62 Suppl 10 :718. 3. Ventura SJ et al. Natl Vital Stat Rep. 2008;56(15):1-25, 28. 4. CDC MMWR 2008; 57(01);1-5
Disclosure:
M. E. B. Clowse,
UCB,
5;
D. C. Wolf,
UCB,
5;
C. Stach,
UCB,
3,
UCB,
1;
G. Kosutic,
UCB,
3,
UCB,
1;
S. Williams,
UCB,
3;
I. Terpstra,
UCB,
1,
UCB,
3;
U. Mahadevan,
UCB,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-of-pregnancy-in-subjects-exposed-to-certolizumab-pegol/